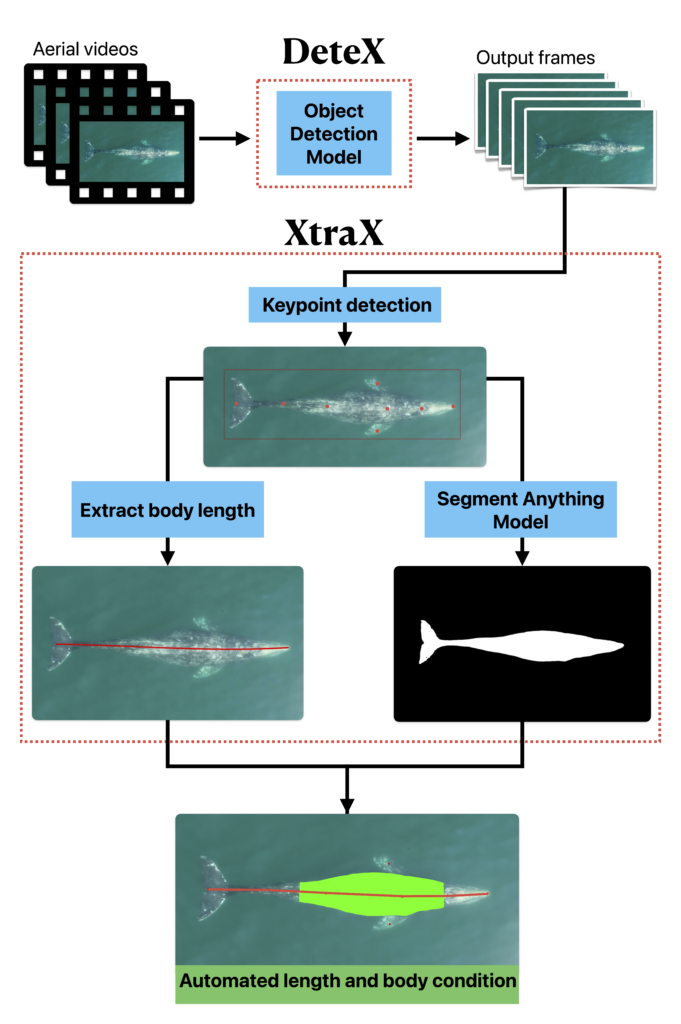
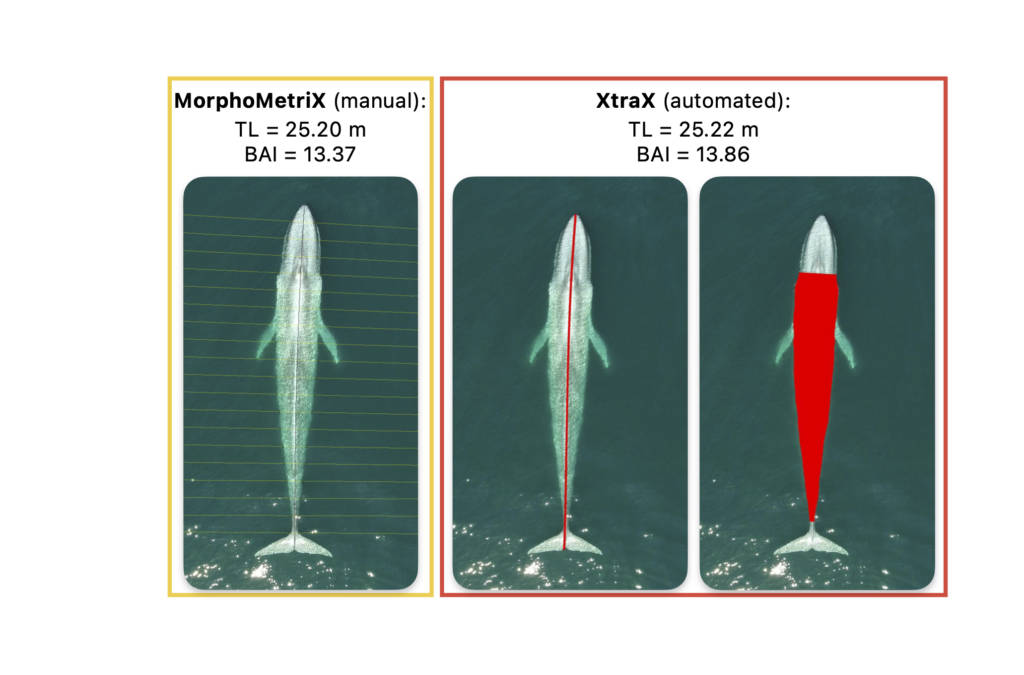
By Natalie Chazal, PhD student, OSU Department of Fisheries, Wildlife, & Conservation Sciences, Geospatial Ecology of Marine Megafauna Lab
Ever noticed how our skin gets pruny and overly soft after just ten minutes in the water? That’s because human skin is adapted for life on dry land, where retaining moisture is a primary concern. In contrast, cetaceans have evolved remarkable adaptations to thrive in the cold, salty ocean water for their entire lives. Understanding cetacean skin is crucial for conservation efforts, as it allows us to monitor and assess the overall health of these marine populations. By analyzing skin conditions, we can identify scarring patterns and lesions that may indicate interactions with human activities, such as entanglements or boat strikes, which can inform more effective risk assessment and mitigation strategies. Additionally, tracking the progression of skin diseases provides vital information on the prevalence and impact of pathogens, in order to guide more targeted management strategies to improve whale health and population resilience in their changing environments. To fully appreciate why monitoring skin diseases in cetaceans matters, let’s first explore the anatomy and physiology of cetacean skin and understand how scarring and diseases occur.
Whale skin has similar layers to our own, but modified over millions of years of evolution. Thicker than terrestrial mammals, the epidermis (the outermost layer) in marine mammals is designed to help maintain hydration in a hyperosmotic (very salty) environment where water is trying to flow into the cells of the whale. This top layer sloughs off at the surface as new cells are continuously renewed. The hypodermis, or blubber layer, is composed of primarily vascularized fat cells which insulate, store energy, and regulate buoyancy (Figure 1).

Some other interesting skin adaptations that allow whales to maximize their efficiency underwater include near hairlessness, no sweat glands, and high levels of melanin. First, cetacean hairlessness helps them reduce drag in the water, but they don’t quite lack all hair. Most species of whales have hair around their mouths when they’re developing in the womb and then lose their hair either before birth or shortly after. Some species, like the humpback, have tubercles that are modified hair follicles to help them sense their surroundings, similar to whiskers on a dog. Second, because sweating is not effective for thermoregulation in the aquatic environment, whales have lost the sweat gland structure in their skin, making it slightly less permeable than terrestrial mammals. Their lack of glands also means that whales don’t secrete their own oils to maintain the moisture of the skin. So, if they’re exposed to dry air, their skin will dry out faster than the skin of terrestrial mammals. Lastly, melanin pigments vary from species to species. You can easily see this when we compare lateral surface photos of different species (Figure 2).

This difference in coloration can be used by animals for camouflage either to avoid predators or to help ambush prey, and helps us to identify the species while they are at the surface. Coloration can also change as an animal ages and can help signal to us or other conspecifics the age or reproductive status of the individual (Caro et al. 2011). The melanin that creates these different colorations can protect whales against the harmful effects of UV radiation by absorbing and dissipating UV radiation, which decreases how far it penetrates into the skin, reducing cell damage (Morales-Guerrero et al. 2017).
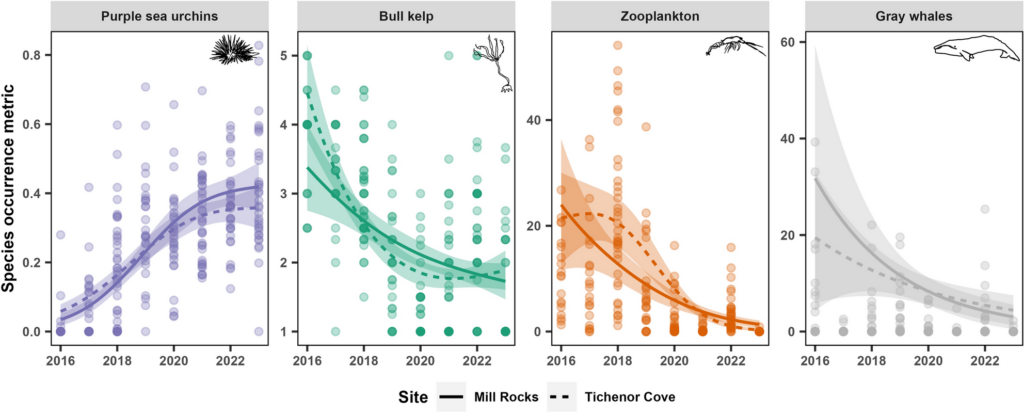
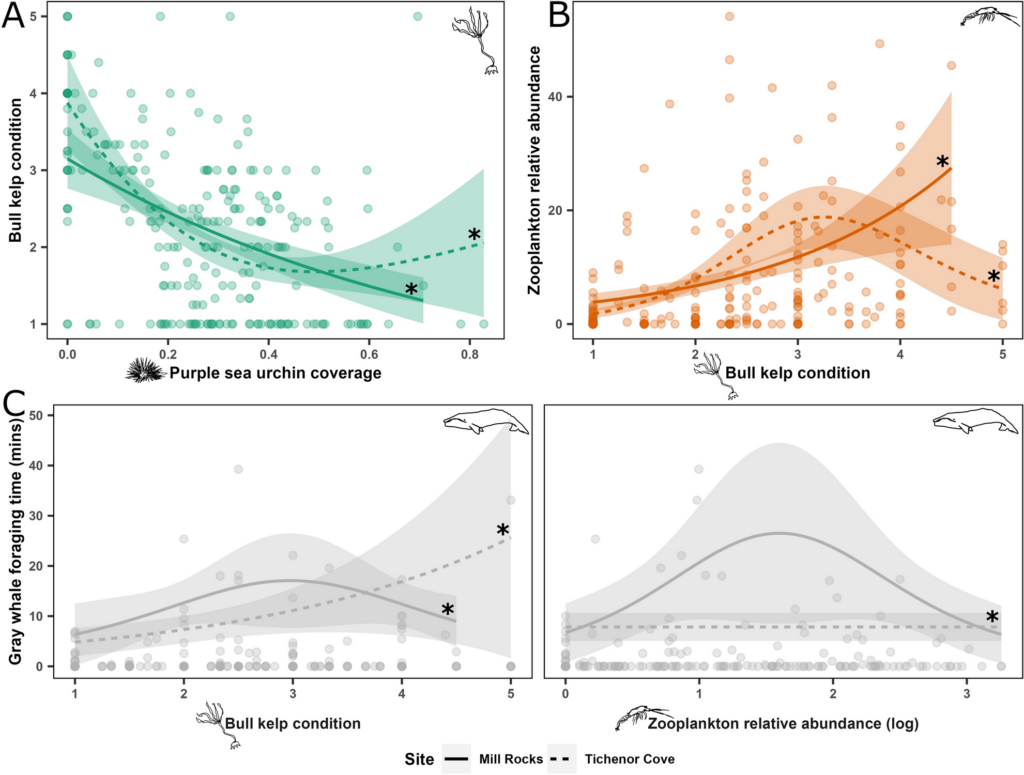
Thus, whale skin is very well adapted to the aquatic environment, from thick blubber layers to no sweat glands. However, despite these adaptations, cetaceans remain vulnerable to a range of pathogens. The major skin diseases documented in whales can fall into 4 categories: viral, bacterial, fungal, and parasitic. Viral infections in cetaceans involve the invasion of host cells, where viruses replicate and cause cell death or dysfunction, leading directly to skin lesions or nodules. Viruses can also manipulate the host immune response, suppressing immunity and exacerbating inflammation, which further contributes to skin damage. In contrast, fungal infections typically involve fungal growth and colonization on the skin surface or within tissues, with some fungi producing toxins that directly damage cells or provoke inflammatory responses (Espregueira et al. 2023). Bacterial infections in cetaceans often result from bacterial invasion and multiplication within skin tissues, accompanied by toxin production that damages cells and triggers a robust inflammatory response (Bressem et al. 2009). Parasitic infections, such as barnacle and whale lice infestations, can cause irritation, abrasions, and compromise the skin’s protective function, leading to localized inflammation and potential secondary infections.
Understanding the specific causes of skin conditions in cetaceans is crucial because different pathogens spread through populations in distinct ways, impacting both individuals and population level health. Viral infections, for instance, can spread rapidly within populations through direct contact or respiratory droplets, potentially leading to widespread outbreaks and systemic effects. Fungal infections may persist in environmental reservoirs (spores of fungus can exist in seawater, sediment, organic marine debris, and the air) and can affect multiple individuals over time, particularly in conditions favoring fungal growth. Bacterial infections often spread through direct contact or contaminated environments, posing risks of localized outbreaks and secondary complications. Parasitic infestations, such as barnacles and whale lice, can transmit between individuals through close contact or shared habitat spaces (Romero et al 2012). By accurately identifying the causative agents of skin diseases, we can assess their transmission dynamics, anticipate population-level impacts, and implement targeted management strategies to mitigate disease spread and preserve whale health.
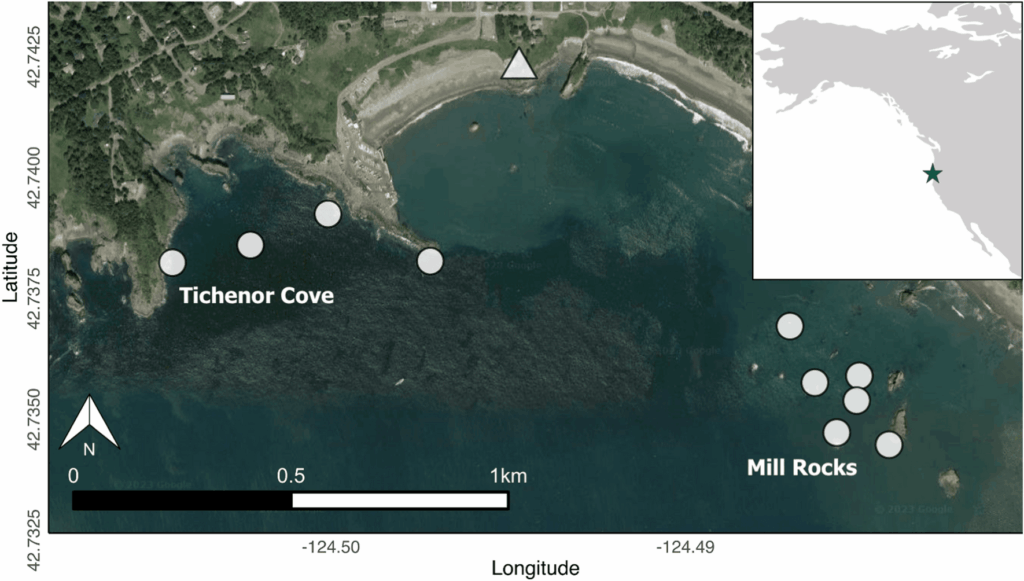
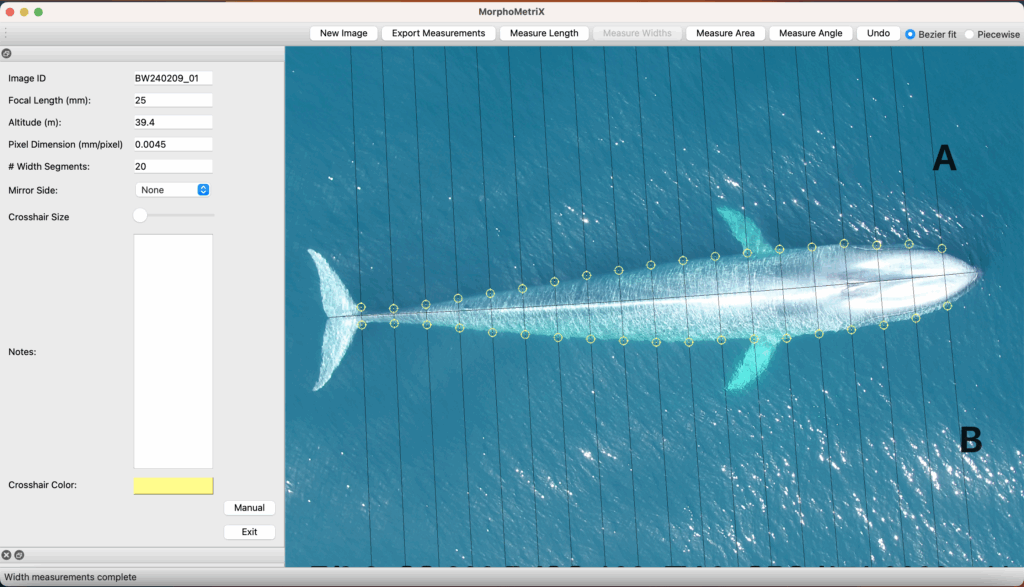
There are complex factors that contribute to skin disease prevalence in cetaceans. Environmental degradation, chemical pollution, climate change, and other anthropogenic stressors are known to lower immune systems, and degrade prey quality and quantity (Bressem et al. 2009). To understand the interactions between disease and the environment, we have to begin by establishing baseline health metrics. This summer, we will characterize an emerging skin disease in gray whales (see Zorro’s progression in Figure 3) using the photographs taken from the last 9 years of GRANITE fieldwork. Gray whales are particularly vulnerable to environmental threats because of their reliance on nearshore habitats. Unlike some other cetacean species that venture into deeper waters, gray whales are primarily coastal dwellers, feeding on benthic and epi-benthic organisms found in shallow, nutrient-rich waters. This dependence on nearshore environments exposes them to numerous risks. Pollution from runoff, oil spills, and plastic debris accumulates in these coastal waters, disrupting their immune systems leaving them more susceptible to disease. Climate change can induce shifts in the environment that alter the availability and quality of these habitats, potentially forcing them into proximity of other animals or places that harbor more disease. Habitat degradation due to coastal development and human activities like overfishing and increased vessel traffic further restricts their access to critical feeding areas (Bressem et al. 2009).

These cumulative impacts increase the susceptibility of gray whales to diseases and stressors, highlighting the urgent need for baseline health assessments and identifying early signs of environmental stress (Stimmelmayr 2020). By documenting and analyzing skin conditions of gray whales through photographs, we can track changes over time and correlate them with environmental factors like pollution levels or habitat alterations. This non-invasive approach not only provides valuable insights into the prevalence and severity of skin diseases but also helps to understand broader ecological health trends in gray whale populations.
P.S. Check out IndividuWhale to explore some great examples of how the skin condition of some of the local Oregon PCFG gray whales compare to each other and how we use their specific markings to help identify them in the field.
References
Barlow, D.R., Pepper, A.L., Torres, L.G., 2019. Skin Deep: An Assessment of New Zealand Blue Whale Skin Condition. Frontiers in Marine Science 6.
Bressem, M.-F.V., Raga, J.A., Guardo, G.D., Jepson, P.D., Duignan, P.J., Siebert, U., Barrett, T., Santos, M.C. de O., Moreno, I.B., Siciliano, S., Aguilar, A., Waerebeek, K.V., 2009. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Diseases of Aquatic Organisms 86, 143–157. https://doi.org/10.3354/dao02101
Callewaert, C., Ravard Helffer, K., Lebaron, P., 2020. Skin Microbiome and its Interplay with the Environment. Am J Clin Dermatol 21, 4–11. https://doi.org/10.1007/s40257-020-00551-x
Caro, T., Beeman, K., Stankowich, T., Whitehead, H., 2011. The functional significance of colouration in cetaceans. Evol Ecol 25, 1231–1245. https://doi.org/10.1007/s10682-011-9479-5
Espregueira Themudo, G., Alves, L.Q., Machado, A.M., Lopes-Marques, M., da Fonseca, R.R., Fonseca, M., Ruivo, R., Castro, L.F.C., 2020. Losing Genes: The Evolutionary Remodeling of Cetacea Skin. Front. Mar. Sci. 7. https://doi.org/10.3389/fmars.2020.592375
Menon, G.K., Elias, P.M., Wakefield, J.S., Crumrine, D., 2022. CETACEAN EPIDERMAL SPECIALIZATION: A REVIEW. Anat Histol Embryol 51, 563–575. https://doi.org/10.1111/ahe.12829
Morales-Guerrero, B., Barragán-Vargas, C., Silva-Rosales, G.R., Ortega-Ortiz, C.D., Gendron, D., Martinez-Levasseur, L.M., Acevedo-Whitehouse, K., 2017. Melanin granules melanophages and a fully-melanized epidermis are common traits of odontocete and mysticete cetaceans. Veterinary Dermatology 28, 213-e50. https://doi.org/10.1111/vde.12392
Mouton, M., Botha, A., Mouton, M., Botha, A., 2012. Cutaneous Lesions in Cetaceans: An Indicator of Ecosystem Status?, in: New Approaches to the Study of Marine Mammals. IntechOpen. https://doi.org/10.5772/54432
Pitman, R.L., Durban, J.W., Joyce, T., Fearnbach, H., Panigada, S., Lauriano, G., 2020. Skin in the game: Epidermal molt as a driver of long-distance migration in whales. Marine Mammal Science 36, 565–594. https://doi.org/10.1111/mms.12661
Romero, A., Keith, E.O., 2012. New Approaches to the Study of Marine Mammals. BoD – Books on Demand.
Stimmelmayr, R., Gulland, F.M.D., 2020. Gray Whale (Eschrichtius robustus) Health and Disease: Review and Future Directions. Frontiers in Marine Science 7.
Su, C.-Y., Hughes, M.W., Liu, T.-Y., Chuong, C.-M., Wang, H.-V., Yang, W.-C., 2022. Defining Wound Healing Progression in Cetacean Skin: Characteristics of Full-Thickness Wound Healing in Fraser’s Dolphins (Lagenodelphis hosei). Animals (Basel) 12, 537. https://doi.org/10.3390/ani12050537
Van Bressem, M.-F., Van Waerebeek, K., Duignan, P.J., 2022. Tattoo Skin Disease in Cetacea: A Review, with New Cases for the Northeast Pacific. Animals 12, 3581. https://doi.org/10.3390/ani12243581