By Dr. Dawn Barlow, Postdoctoral Scholar, OSU Department of Fisheries, Wildlife, and Conservation Sciences, Geospatial Ecology of Marine Megafauna Lab
The SAPPHIRE project’s inaugural 2024 field season has officially wrapped up, and the team is back on shore after an unexpected but ultimately fruitful research cruise. The project aims to understand the impacts of climate change on blue whales and krill, by investigating their health under variable environmental conditions. In order to assess their health, however, a crucial first step is required: finding krill, and finding whales. The South Taranaki Bight (STB) is a known foraging ground where blue whales typically feed on krill found in the cool and productive upwelled waters. This year, however, both krill and blue whales were notoriously absent from the STB, leaving us puzzled as we compulsively searched the region in between periods of unworkable weather (including an aerial survey one afternoon).

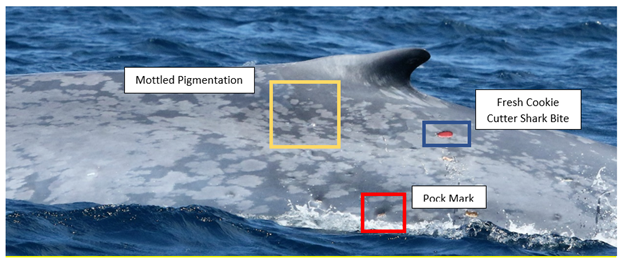
The tables felt like they were turning when we finally found a blue whale off the west coast of the South Island, and were able to successfully fly the drone to collect body condition information, and collect a fecal sample for genetic and hormone analysis. Then, we returned to the same pattern. Days of waiting for a weather window in between fierce winds, alternating with days of searching and searching, with no blue whales or krill to be found. Photogrammetry measurements of our drone data over the one blue whale we found determined it to be quite small (only ~17 m) and in poor body condition. The only krill we were able to find and collect were small and sparsely mixed in to a massive gelatinous swarm of salps. Where were the whales? Where was their prey?

Then, a turn of events. A news story with the headline “Acres of krill washing up on the coastline” made its way to our inboxes and news feeds. The location? Kaikoura. On the other side of the Cook Strait, along the east coast of the South Island. With good survey coverage in the STB resulting in essentially no appearances of our study species, this report of krill presence along with a workable weather forecast in the Kaikoura area had our attention. In a flurry of quick decision-making (Leigh to Captain: “Can we physically get there?” Captain to Leigh: “Yes, we can.” Leigh to Captain: “Let’s go.”), we turned the vessel around and surfed the swells to the southeast at high speed.

Twelve hours later we arrived at dusk and anchored off the small town of Kaikoura, with plans to conduct a net tow for krill before dawn the next morning. But the krill came to us! In the wee hours of the morning, the research vessel was surrounded by swarming krill. The dense aggregation made the water appear soup-like, and attracted a school of hungry barracuda. These abundant krill were just what was needed to run respiration experiments on the deck, and to collect samples to analyze their calories, proteins, and lipids back in the lab.

With krill in the area, we were anxious to find their blue whale predators, too. Once we began our visual survey effort, we were alerted by local whale watchers of a blue whale sighting. We headed straight to this location and got to work. The day that followed featured another round of krill experiments, and a few more blue whale sightings. Predator and prey were both present, a stark contrast to our experience in the previous weeks within the STB and along the west coast of the South Island. The science team and crew of the R/V Star Keys fell right into gear, carefully maneuvering around these ocean giants to collect identification photos, drone flights, and fecal samples, finding our rhythm in what we came here to do. We are deeply grateful to the regional managers, local Iwi representatives, researchers, and tourism operators that supported making our time in Kaikoura so fruitful, on just a moment’s notice.

What does it all mean? It’s hard to say right now, but time and data analysis will hopefully tell. While this field season was certainly unexpected, it was valuable in many ways. Our experiences this year emphasize the pay-off of being adaptable in the field to maximize time, money, and data collection efforts (during our three-week cruise we slept in 10 different ports or anchorages, did an aerial survey, and rapidly changed our planned study area). Oftentimes, the cases that initially “don’t make sense” are the ones that end up providing key insights into larger patterns. No doubt this was a challenging and at times frustrating field season, but it could also be the year that provides the greatest insights. After two more years of data collection, it will be fascinating to compare this year’s blue whale and krill data in the greater context of environmental variability.

One thing is clear, the oceans are without question already experiencing the impacts of global climate change. This year solidified the importance of our research, emphasizing the need to understand how krill—a crucial marine prey item—and their predators are being affected by warming and shifting oceans.

Did you enjoy this blog? Want to learn more about marine life, research, and conservation? Subscribe to our blog and get a weekly alert when we make a new post! Just add your name into the subscribe box below!