By Nadia Leal, GEMM Lab summer intern, OSU senior, Department of Fisheries, Wildlife, and Conservation Sciences
The OSU GEMM Lab gray whale foraging ecology project in Port Orford is in its seventh year of research. I have the honor to serve as a field assistant for the project as part of Team “Heck Yeah” for the summer 2021 field season. In doing so, I have been presented with the opportunity to take part in its enduring legacy. It is a legacy characterized by novel discovery, distinguished leadership, and endless adventure. These particular aspects motivated me to pursue this internship. Further, the desire to seek out gray whales (Eschrichtius robustus) — a species epitomizing the ability to exhibit resilience in the face of adversity after having experienced two unusual mortality events (UME) in the past two decades and having recovered from historically low population abundances due to whaling — sparked immeasurable excitement.

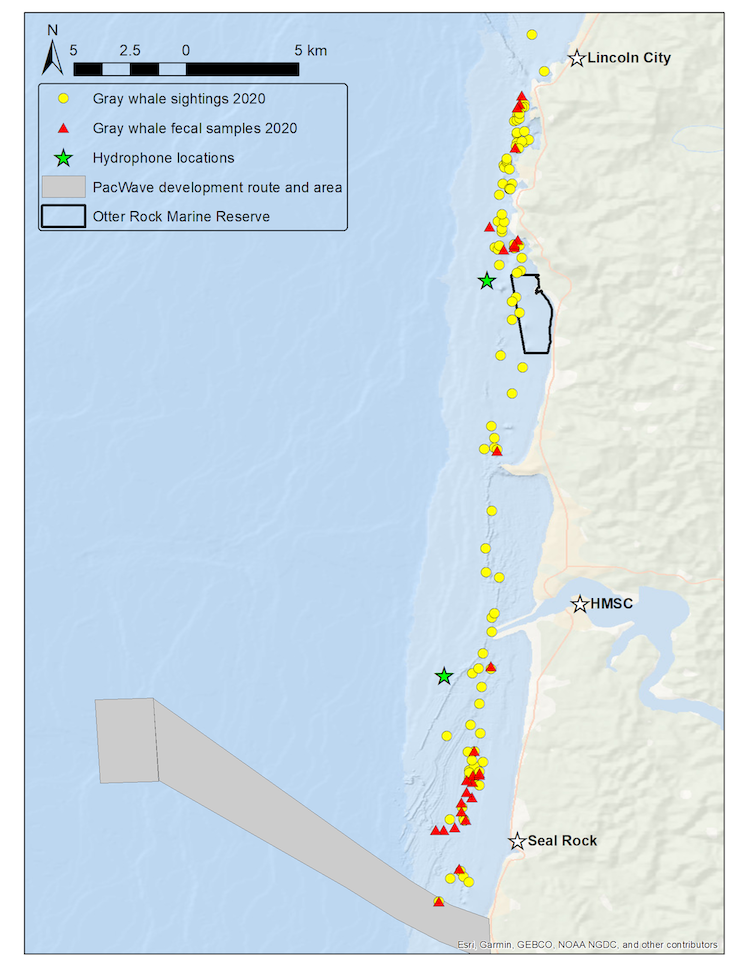
The skills we are acquiring during this field season are essential to master so that I can pursue my aspirations of becoming a marine conservation biologist. For example, we have learned how to operate a theodolite, which is a surveying tool used regularly in marine mammal research to accurately calculate the location of cetaceans and track their movements (Figure 1). We are also learning how to operate a number of other research equipment, to navigate a tandem kayak using a GPS, to process various forms of data, and to identify gray whales! I have especially enjoyed collecting prey samples and navigating our tandem kayak, as kayaking is a summer tradition for my family and the opportunity to kayak in this context is certainly the high point of this internship. The kayak is named “Robustus” after the scientific name of the gray whale: Eschrichtius robustus! (Figure 2).
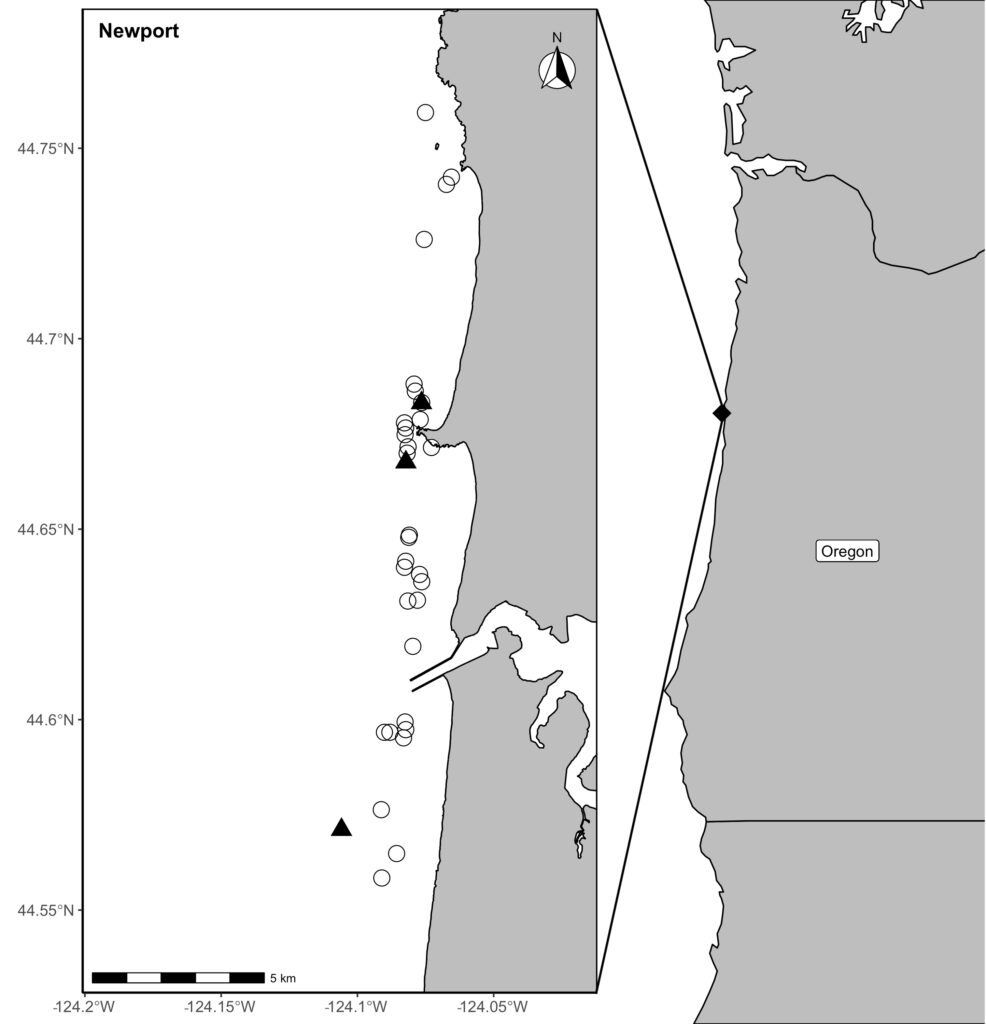
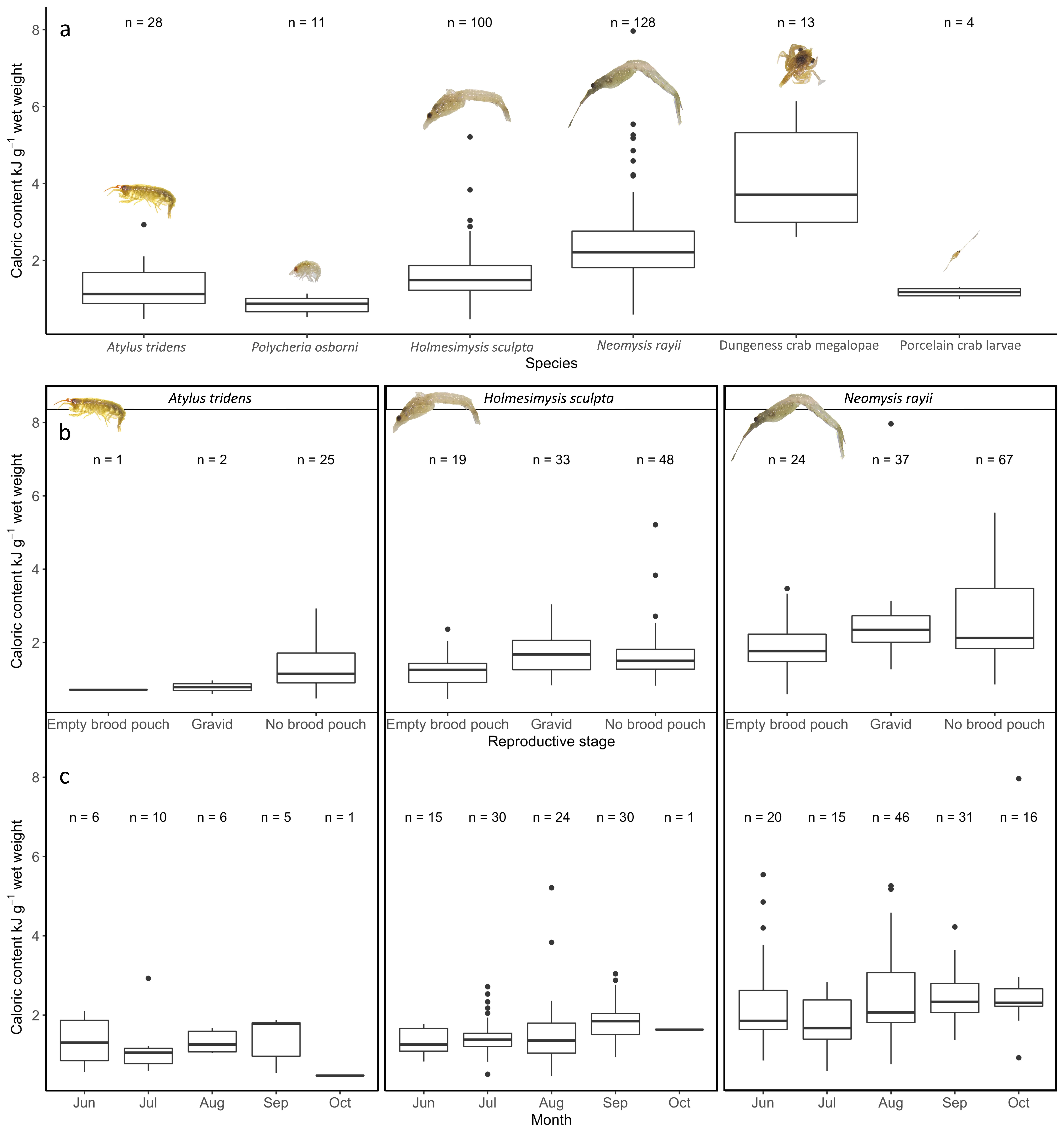
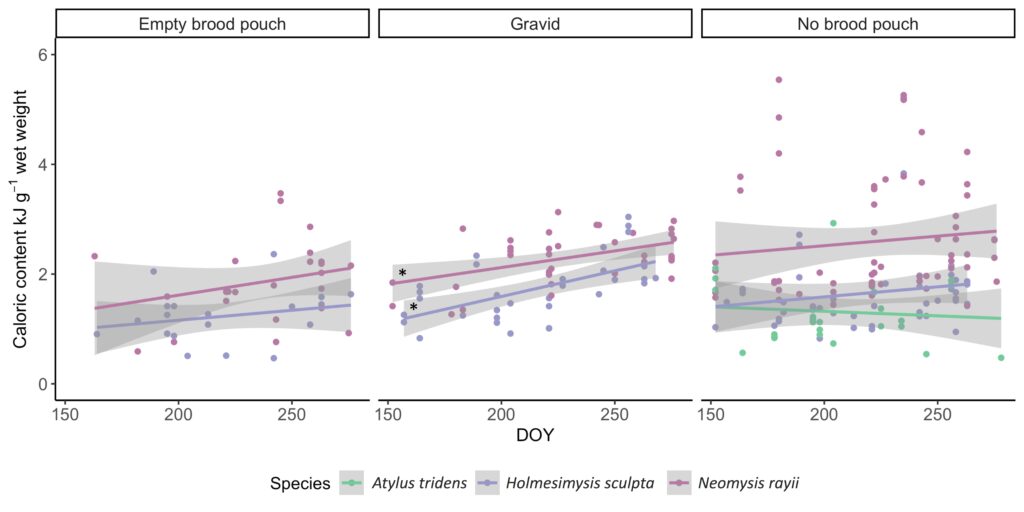
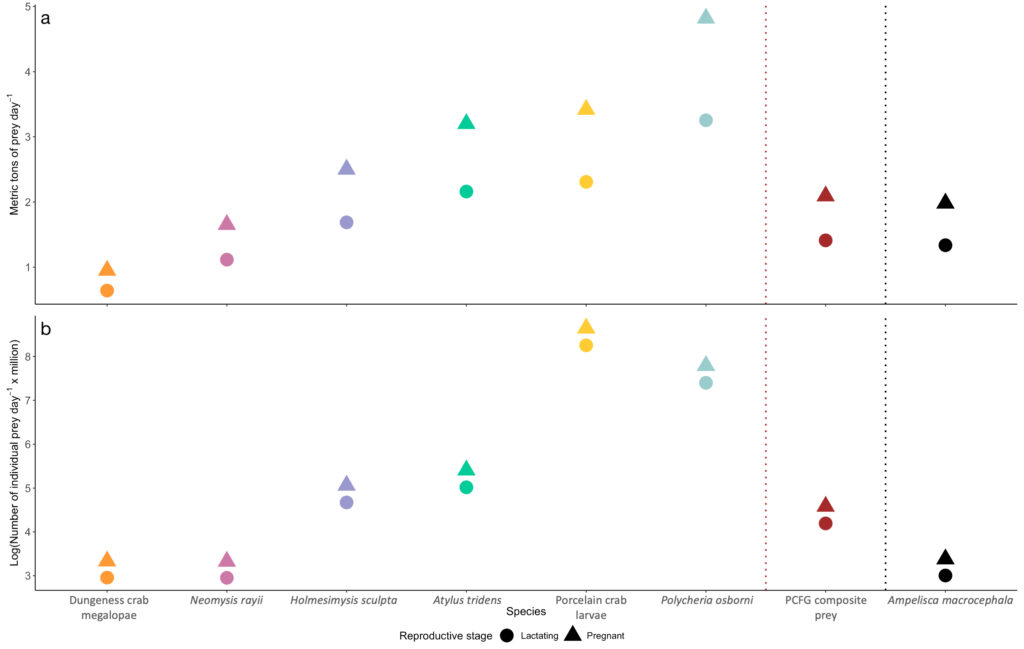
The Port Orford project aims to determine how gray whale foraging is affected by prey quantity and quality. In fact, gray whales exhibit specificity in their selection of prey on the basis of caloric content (Hildebrand 2020). I am particularly interested in the underlying implications these findings imply: the notion of conscious reasoning and decision-making by individual whales as they seek the most suitable prey for its dietary needs among other options to maximize its survivability. Are gray whales in possession of an awareness that allows them to exhibit intentional preference? Can the behavior be attributed to instinct and/or learned behavior, or to cognition comparable to human preference? These and similar questions are my motivation for studying the realm of marine mammal biology. These questions concern intelligence and evolution, which can be effectively investigated through an analysis of cetacean brain structure, as it likely has compelling relationships to their extensive behavioral abilities (Hof and Van Der Gucht 2007).
For instance, the brain of the gray whale has expanded and developed extensively over evolutionary time in response to distinct selection pressures. Evidence affirms that the behavioral challenges associated with foraging exert strong selection pressures on the evolution of their brain size and structure (Muller and Montgomery 2019)! Selection pressures associated with social cognition are also believed to have contributed to such growth (Connor et al. 1998; Marino 2002; Shultz and Dunbar 2010 ). Further, their neural organization has increased in complexity, leading to greater function and usage of the cortical portion of the brain, which is the portion responsible for higher level activity (Oelschläger and Oelschläger 2002).
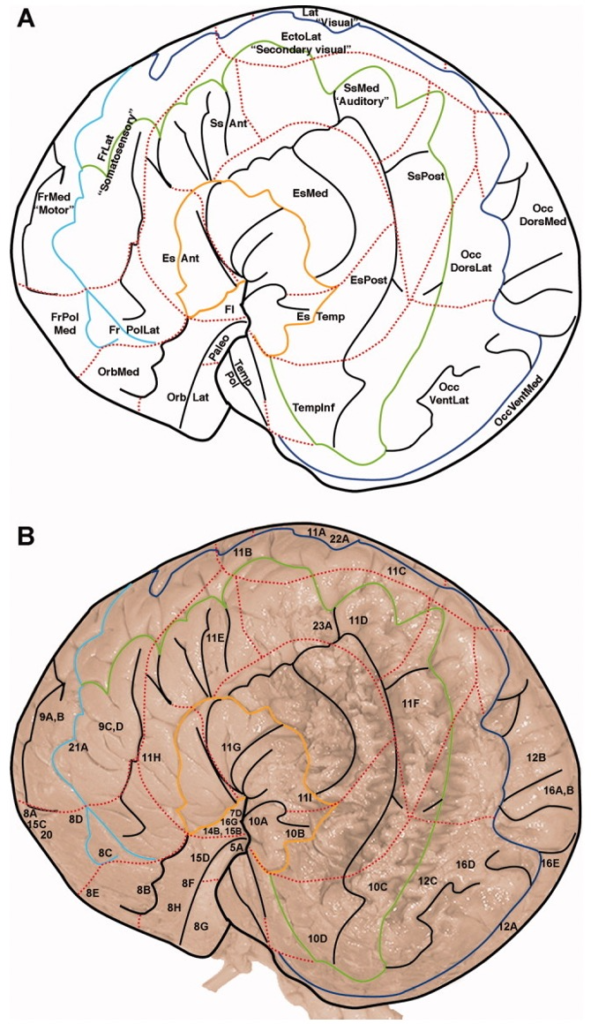
Though research about baleen whale brain morphology is not as pervasive as that of toothed whales (due to increased susceptibility of toothed whales to captivity given the feasibility of their capture and subsequent analysis in lab/controlled setting), studies have indicated that the brain of baleen whales share similarities to those of humans (Wade et. al 2012). In particular, similarities exist in the frontal lobe of the brain, which is responsible for the complex activities of self-awareness, reasoning, and behavior, as well as for problem-solving and motivation (Hof and Van Der Gucht 2007) (Figure 3). These findings indicate that baleen whales, including the gray whale, have the capability to exhibit intentional preference and take part in conscious decision-making in the recognition of different prey species. The mechanisms responsible for how gray whales may discern prey likely involve a number of the sensory systems, differing in respect to spatial scale (Torres 2017). Thus, gray whales likely rely on various sensory methods, such as vision, sound perception/reception, chemoreception, or an oceanographic stimulus, at differing scales to locate and discern prey. The sensory method employed is dependent on their distance from prey.
Though we cannot yet confirm whether and/or how gray whales are capable of distinguishing between prey species, what is certain, is that the gray whale is intelligent and quite similar to us. Moreover, they are representative of strength and endurance, providing lessons we can learn from and qualities we can embody. Despite the threats to the species from fishing gear entanglement, ship collisions, climate change, oil industry developments, and being historically hunted, they have remarkably persisted. Thus, we must ensure the existence of the gray whale so they too may thrive for the rest of time, with healthy lives and habitat that is rightfully theirs.
P.S. I would like to thank the GEMM Lab, Oregon State University, Shalynn Pack, Port Orford Sustainable Seafood, Port Orford Co-op, South Coast Tours, Nicki’s Knick Knacks, Leigh Torres, Lisa Hildebrand, Allison Dawn, Clara Bird, Tom Calvanese, Maddie English, Jasen White, and Damian Amerman-Smith for making the internship as special and memorable as it is/was.
References
Connor, R. C., Mann, J., Tyack, P. L., and Whitehead, H. (1998). Social evolution in toothed whales. Trends in Ecology and Evolution, 13(6): 228– 232. doi: https://doi.org/10.1016/S0169‐5347(98)01326‐3
Hildebrand, L. (2020). Tonight’s specials include mysids, amphipods, and more: an examination of the zooplankton prey of Oregon gray whales and its impact on foraging choices and prey selection. Master’s thesis, Oregon State University.
Hof, P.R., and Van Der Gucht, E. (2007). Structure of the cerebral cortex of the humpback whale, Megaptera novaengliae(Cetacea, Mysticeti, Balaenopteridae). The Anatomical Record 290:1-31 doi: 10.1002/ar.a.20407
Marino, L. (2002). Convergence of complex cognitive abilities in cetaceans and primates. Brain, Behavior, and Evolution59: 21–32. doi: https://doi. org/10.1159/000063731
Oelschläger, H.A., and Oelschläger, J.S. (2002). Brains. In: Perrin WF, Wu¨ rsig B, Thewissen JGM, editors. Encyclopedia of marine mammals. San Diego, CA: Academic Press. p 133–158.
Shultz, S., & Dunbar, R. (2010). Encephalization is not a universal macroevolutionary phenomenon in mammals but is associated with sociality. Proceedings of the National Academy of Sciences of the United States of America 107(50): 21582–21586. doi: https://doi.org/10.1073/ pnas.1005246107
Torres, L.G. (2017). A sense of scale: foraging cetaceans’ use of scale-dependent multimodal sensory systems. Marine Mammal Science 33: 1170-1193. doi: 10.1111/mms.12426
Wade, P.R., Reeves, R.R., and Mesnick, S.L. (2012). Social and behavioral factors in cetacean responses to overexploitation: are odontocetes less “resilient” than mysticetes?. The Journal of Marine Biology 2012: 1-15. doi:10.1155/2012/567276