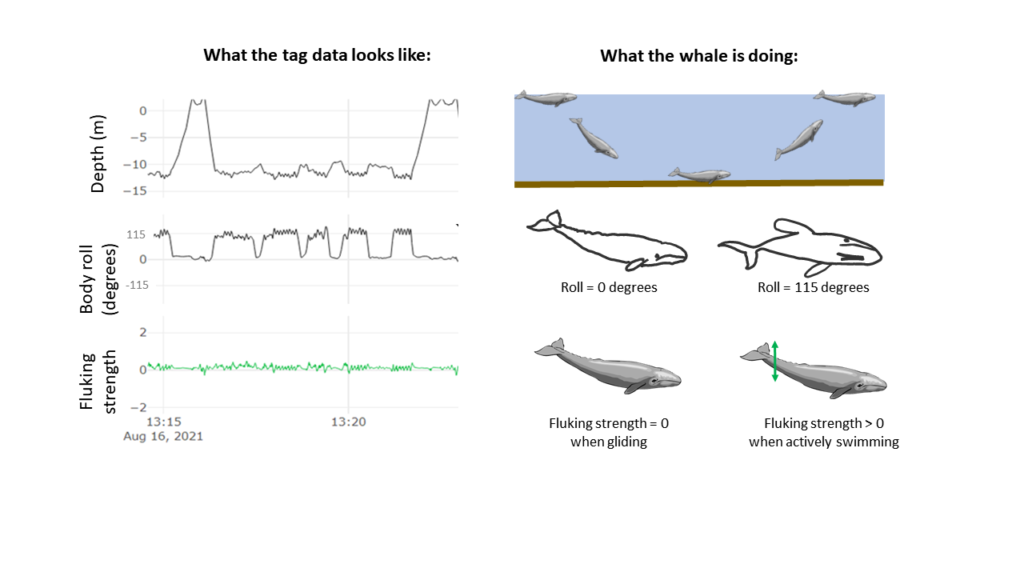
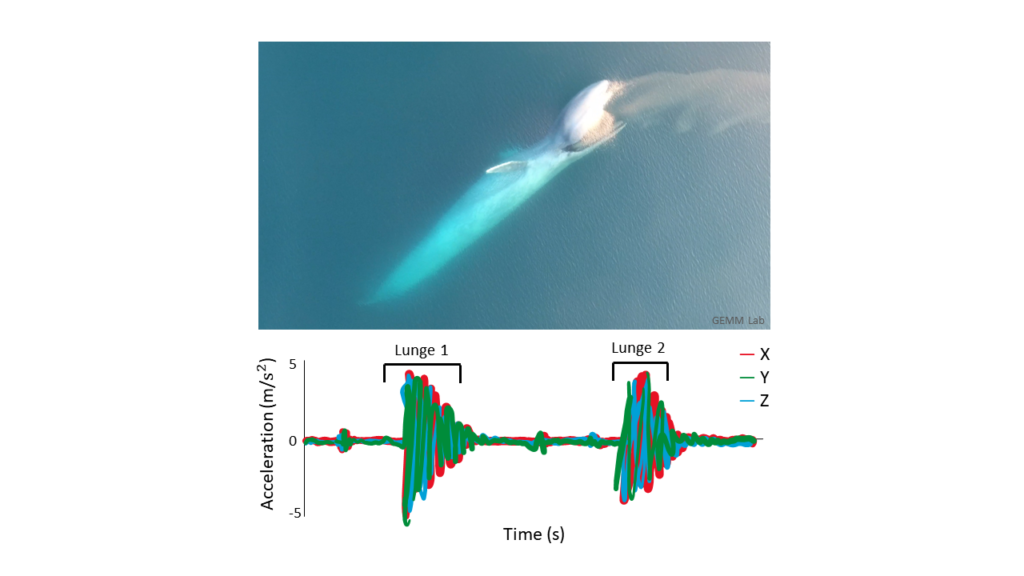
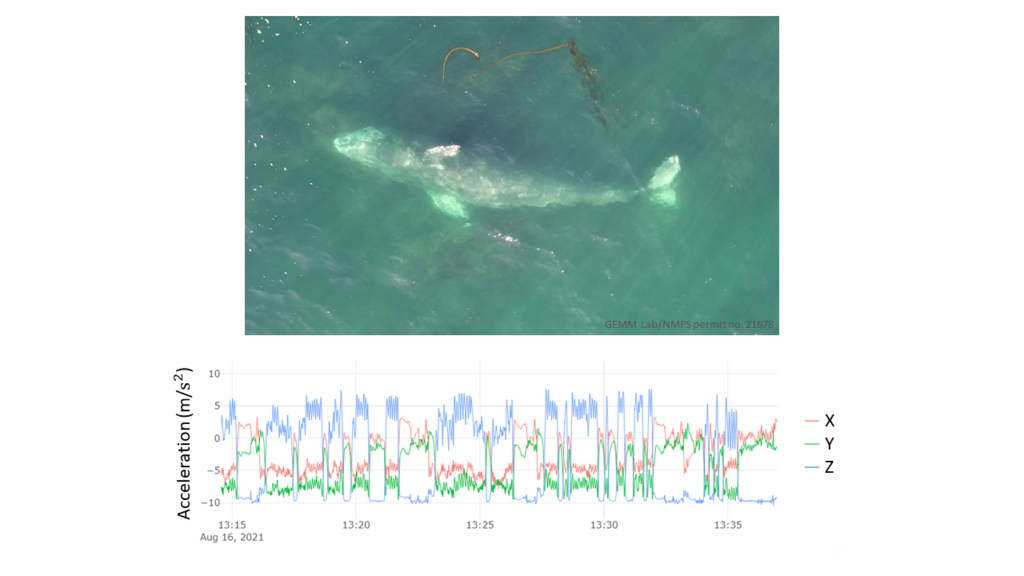
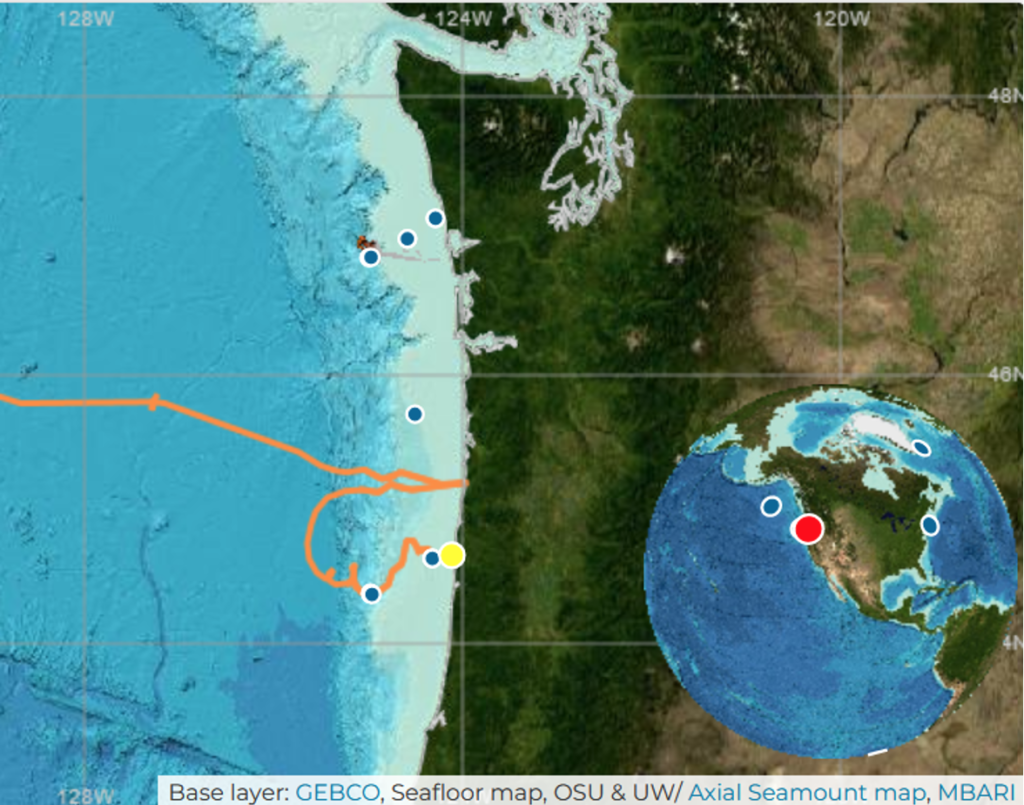
Dr. Alejandro A. Fernández Ajó, Postdoctoral Scholar, Marine Mammal Institute – OSU Department of Fisheries, Wildlife, & Conservation Sciences, Geospatial Ecology of Marine Megafauna (GEMM) Lab.
The English language is recognized as the international language of science (Gordin, 2015); I believe this is a useful convention that allows scientists to communicate ideas and gain access to global scientific literature regardless of their origin or native tongue. However, this avenue for sharing knowledge is open only for those proficient in English, and many scientists and users of scientific information, such as policy makers and conservationists, communicate on a daily basis in languages other than English. This inevitably creates barriers to the transfer of knowledge between communities, potentially impacting conservation and management because scientific knowledge is often unavailable in local languages.
Although in non-English speaking countries, local journals are receptive to publishing scientific research in languages other than English (i.e., their local language), oftentimes these local journals are perceived as low-quality and have a relatively low impact factor, making publishing in such journals less attractive to scientists. Therefore, readers with language barriers only have access to limited studies and are often unaware of the most significant research, even when the research is conducted in their region. This situation can result in a void of information relevant for environmental policies and conservation strategies. Ensuring that research findings are available in the local language of the region in which the research is conducted is an important step in science communication, but one that is often neglected.
In addition, scientists with English as a Foreign Language (EFL) confront the added challenge of navigating a second language while writing manuscripts, preparing and presenting oral presentations, and developing outreach communications (Ramirez-Castaneda, 2020). For example, EFL researchers have reported that one of the primary targets of criticism for their manuscripts under review is often the quality of their English rather than the science itself (Drubin and Kellogg, 2012). In academia, most job interviews and PhD applications are conducted in English; and grant and project proposals are often required to be written in English, which can be particularly challenging and can impact the allocation of resources for research and conservation in non-English speaking regions.
I am from Argentina, and I am a native Spanish speaker. I am fortunate to have started learning English at an early age and continue practicing with international collaborations and traveling abroad. Being able to communicate in English has opened many doors for me, but I recognize that I am in a privileged position with respect to many Argentinians and South Americans in general, where the majority of the population receives minimal training in English and bilingualism with English is very low. Thus, socioeconomic status can influence English proficiency, which then determines scientific success and access to knowledge. I believe that the scientific community should be aware of these issues and work towards improving equality in the process of research collaborations. Providing opportunities for students, and enhancing the availability of scientific knowledge for non-English speaking communities, particularly when the research is relevant for such communities.

Fortunately, there are several examples pointing towards improving equality in the scientific process, access to knowledge, and opportunities for EFL communities in STEM careers. Several journals are now accepting, or considering to accept the publication of papers in multiple languages. One example of this is the journal Integrative Organismal Biology, which provides the option for publishing the paper abstract in multiple languages. In our recent publication, “Male Bowhead Whale Reproductive Histories Inferred from Baleen Testosterone and Stable Isotopes,” we provided an abstract in five different languages, including Inuktitut, one of primary languages of indigenous groups in the area. And, international exchange programs like the Fulbright Foreign Student Program, of which I was a beneficiary between 2018-2020, enable graduate students and young professionals from abroad to study and conduct research in the United States.
In an effort to contribute to addressing these problems, I am working with a group of colleagues from Argentina (María Constanza (Kata) Marchesi and Tomas Marina) to develop graduate level coursework that will be offered at the Universidad Nacional de la Patagonia in Puerto Madryn, Argentina, with the objective to enable students to learn effective communication using English in the scientific environment. Unfortunately, these types of programs focused on EFL proficiency for STEM students are currently rare in Argentina, but my hope is that our work can spur the creation of additional programs for EFL students in STEM across the region.
I want to finish this post with the acknowledgement of the huge support I have form the GEMM Lab, which welcomes diversity, equity, and inclusivity, and promotes a culture of anti-racism, transparency, and acceptance (See the GEMM Lab DEI statement here).
Did you enjoy this blog? Want to learn more about marine life, research and conservation? Subscribe to our blog and get weekly updates and more! Just add your name into the subscribe box below!
References and Additional Readings
Gordin, M. D. (2015). Scientific Babel : How Science Was Done Before and After Global English. Chicago, IL: University of Chicago Press.
Ramírez-Castañeda V (2020) Disadvantages in preparing and publishing scientific papers caused by the dominance of the English language in science: The case of Colombian researchers in biological sciences. PLoS ONE 15(9): e0238372. https://doi.org/10.1371/journal.pone.0238372
Drubin, D. G., and Kellogg, D. R. (2012). English as the universal language of science: opportunities and challenges. Mol. Biol. Cell 23:1399. doi: 10.1091/mbc.E12-02-0108
Amano, T., González-Varo, J. P., & Sutherland, W. J. (2016). Languages are still a major barrier to global science. PLoS Biology, 14(12), e2000933. https://doi.org/10.1371/journal.pbio.2000933
Marden, E., Abbott, R. J., Austerlitz, F., Ortiz-Barrientos, D., Rieseberg, L. H. (2021). Sharing and reporting benefits from biodiversity research. Molecular Ecology, 30(5), 1103–1107. https://doi.org/10.1111/mec.15702
Márquez, M. C., & Porras, A. M. (2020). Science communication in multiple languages Is critical to Its effectiveness. Frontiers in Communication, 5(May). https://doi.org/10.3389/fcomm.2020.00031
Ramírez-Castañeda V (2020) Disadvantages in preparing and publishing scientific papers caused by the dominance of the English language in science: The case of Colombian researchers in biological sciences. PLoS ONE 15(9): e0238372. https://doi.org/10.1371/journal.pone.0238372
Trisos, C. H., Auerbach, J., & Katti, M. (2021). Decoloniality and anti-oppressive practices for a more ethical ecology. Nature Ecology and Evolution, 5(9), 1205–1212. https://doi.org/10.1038/s41559-021-01460-w
Woolston, C, & Osório, J. (2019). When English is not your mother tongue. Nature 570, 265-267. https://doi.org/10.1038/d41586-019-01797-0
Letter to the Editor of Marine Mammal Science: Enhancing the impact and inclusivity of research by embracing multi-lingual science communication (2022) DOI: 10.13140/RG.2.2.29934.08001 http://dx.doi.org/10.13140/RG.2.2.29934.08001
Leal, J. S., Soares, B., Franco, A. C. S., de Sá Ferreira Lima, R. G., Baker, K., & Griffiths, M. (2022). Decolonizing ecological research: a debate between global North geographers and global South field ecologists. https://doi.org/10.31235/osf.io/wbzh2