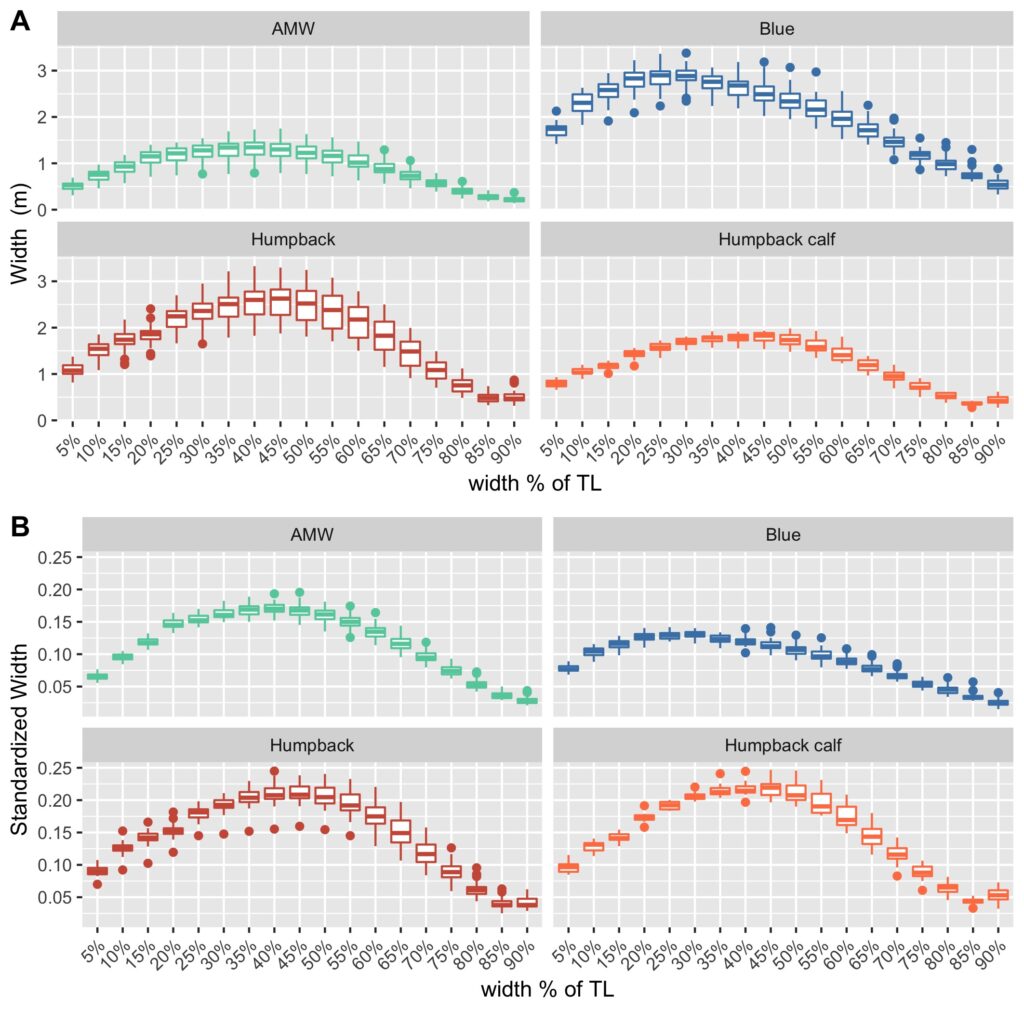
Imogen Lucciano, Graduate student, OSU Department of Fisheries, Wildlife, & Conservation Sciences, Geospatial Ecology of Marine Megafauna Lab.

Pursuing a graduate degree as a member of the Marine Mammal Institute (MMI) comes with many advantages. Developing associations with curious, industrious researchers and working with advanced technological methods are certainly two of them. Particularly, as a member of the HALO project, I have the pleasure of working alongside not only the GEMM’s, but also acoustician Dr. Holger Klinck and his bioacoustics team at the K. Lisa Yang Center for Conservation Bioacoustics at the Cornell Lab (CCB) who have made significant contributions to advance the field for marine mammal research.
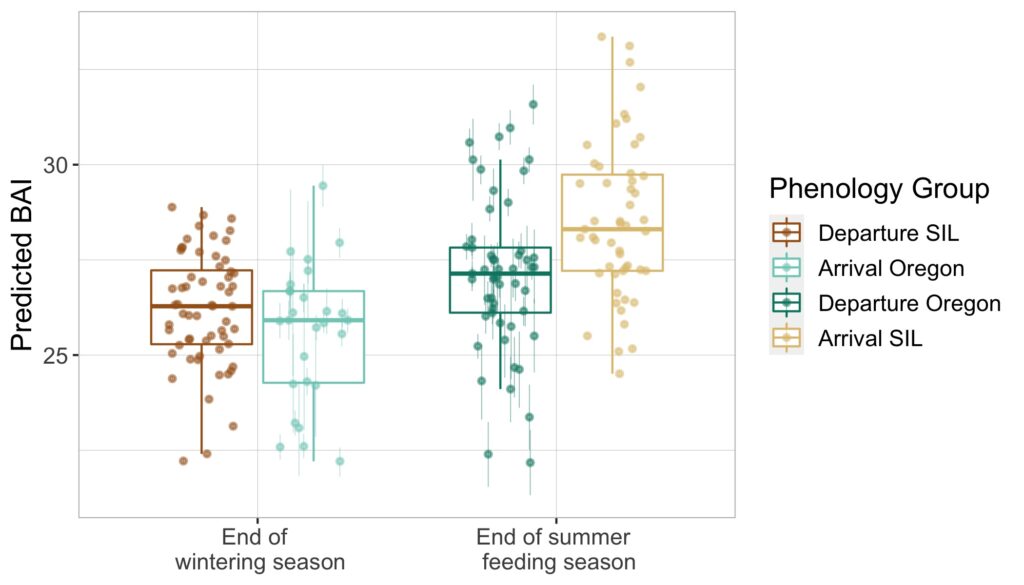
When the HALO project kicked off in October, 2021, Holger and graduate student, Marissa Garcia, arrived for our initial voyage off the Oregon coast with three specialized acoustic recording devices, called Rockhoppers. We deployed each Rockhopper at their designated locations, where they will remain and be replaced every six months, to collect continuous passive acoustic data of cetacean vocalizations. These data are significant because they gather information on all vocalizing whales and dolphins within a detectable range of the Rockhoppers, supporting not only my thesis work concerning fin whale distribution in the Northern California Current (NCC) but has the potential to inform multiple other research projects as well.

Passive acoustic monitoring (PAM) is a non-invasive underwater method of recording acoustic output of cetaceans (Zimmer, 2011), and the Rockhopper is specialized for this task. The Rockhopper relatively small (each weighing ~90lbs.) and can be easily deployed with a minimal team from almost any vessel (Fig 1). The mooring is a simple system that anchors the Rockhopper to the sea floor after it sinks through the water column, tolerating depths up to 3,500 m (Klinck et al., 2020). The device can stay on the ocean floor for up to seven months continuously collecting high-frequency data (up to 197 kHz, 24 bits; Klinck et al., 2020). To recover the Rockhopper, the mooring system (Fig 2) includes an acoustic release; when the correct acoustic signal is transmitted by scientists from the vessel and received down at the seafloor, the Rockhopper is released. It’s positive buoyancy allows it to float to the surface where it is recovered. By developing the Rockhopper with these capabilities, the bioacoustics team at Cornell University have taken several steps to enhance cetacean research.
According to one of it’s designers, David Winiarski, the Rockhopper development team, consisting of himself, Holger Klinck, Raymond Mack, Christopher Tessaglia-Hymes, Dmitri Ponirakis, Peter Dugan, Christopher Jones, and Haru Matsumoto, initiated it’s construction in 2015. Winiarski states that Jones developed the Rockhopper’s initial PAM electronics at Embedded Ocean Systems (EOS), Boston, MA and then the rest of the team developed the remainder of the device in 2017. The Rockhopper contains the electronic system and a 10.8 V Lithium battery pack in an oil-filled Vitrovex 43 cm glass sphere that is encased in hard polyethelene. Two 64 GB memory cards store the collected acoustic data. About every hour the internal processing unit moves the data to two 4 Terabyte solid-state drives in a process that ensures the data is not lost (Klinck et al., 2020). Winiarski attests that it was quite a hectic process to get six complete Rockhoppers ready for their initial deployment, however the team succeeded and in May 2018 they were deployed in the Gulf of Mexico. The Rockhoppers were recovered in 2019 after six months, returning an amazing 21,522 hours of continuous acoustic data (Klinck et al., 2020).
Learning this information about the acoustic devices that will be responsible for collecting my Master’s thesis data is encouraging. I am eager to see the fin whale energy captured within the Rockhopper records. The HALO team, along with myself, Holger, and Marissa, will head back out off the Oregon coast to retrieve our three HALO-designated Rockhoppers in early June (next month). We will then spend the summer at Cornell reading through our first six months of data.
So, why call this acoustic device, the “Rockhopper”? Winiarski explained that since the CCB is a subsect of the Cornell Lab of Ornithology their projects tend to be named after birds. The Rockhopper team thought that this device should respectively be named after a cool marine megafauna. Hence the rockhopper penguin was chosen. I do agree that such an outstanding device is well suited in relation with an equally remarkable marine species.



Left: Rockhopper penguins on a New Zealand hillside. https://nzbirdsonline.org.nz/species/eastern-rockhopper-penguin Upper right: Chris Tessaglia-Hymes and David Winiarski with a Rockhopper acoustic device. Lower right: The first six complete Rockhopper acoustic devices developed at the Cornell Center of Bioacoustics in 2017.
Did you enjoy this blog? Want to learn more about marine life, research, and conservation? Subscribe to our blog and get weekly updates and more! Just add your name into the subscribe box on the left panel.
References
Klinck, H., Winiarski, D., Mack, R., Tessaglia-Hymes, C., Ponirakis, D., Dugan, P., Jones, C., Matsumoto, H. 2020. The Rockhopper: a compact and extensible marine autonomous passive acoustic recording system,” Global Oceans 2020: Singapore – U.S. Gulf Coast: 1-7. https://ieeexplore.ieee.org/document/9388970.
Zimmer, W. 2011. Passive acoustic monitoring of cetaceans. Cambridge University Press, Cambridge, UK.