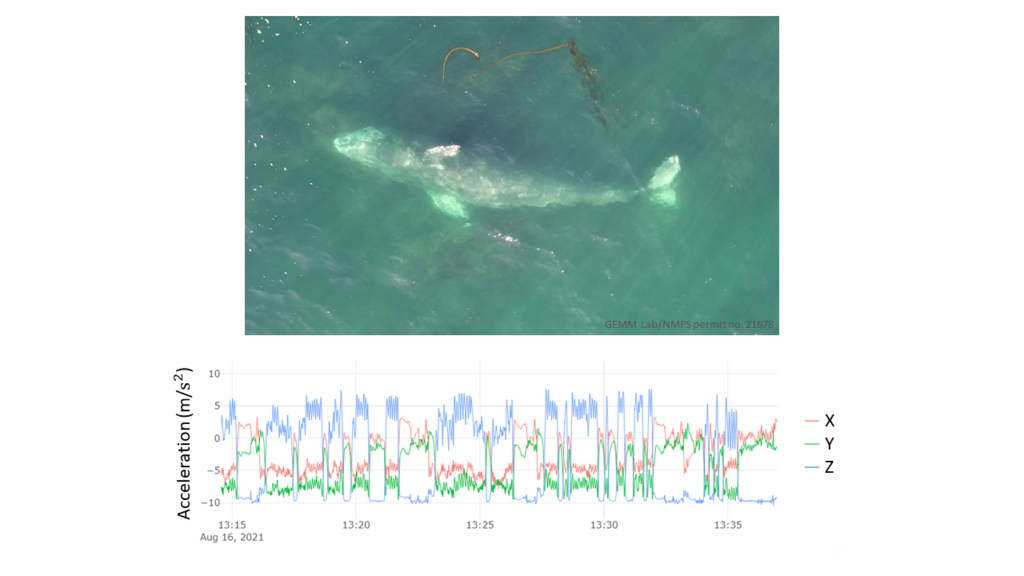
Imogen Lucciano, Graduate student, OSU Department of Fisheries, Wildlife, & Conservation Sciences, Geospatial Ecology of Marine Megafauna Lab.

One year ago, I packed up my 11-year-old daughter, Mavis (for the purposes of this blog, I’ll refer to her as “my sidekick”), our two dogs, and all our books and we moved to Oregon. I was thrilled to arrive and begin my graduate studies in cetacean ecology and bioacoutics with the GEMM lab and the Marine Mammal Institute. It has not been an easy set of tasks to achieve high standards in graduate school while maintaining a constant presence as a single mother, but I am honestly having the time of my life. I am involved in an amazing graduate program and I get to do it with my sidekick cheering me on and making my life feel very whole. This is why I am excited to write this blog reporting on the progression of my thesis and the incredible animals that I have the pleasure of studying: the fin whale.
Fin whales (Balaenoptera physalus) are the second largest cetacean on the planet and are present in nearly all temperate and polar oceanic regions of the world (1). For my master’s thesis, I will focus solely on the fin whales within a detectable range of our team’s research area off the Oregon coast. In the Northern Hemisphere, fin whales are known to grow up to 23 meters in length and weigh up to 40-50 metric tons (2). They have a slender profile and can be further identified by their hook-shaped dorsal fin in addition to a V-shape on their back referred to as a “chevron” (Fig. 1). Fin whales are a baleen whale in the rorqual family, which have adapted lunge feeding as their primary foraging method (3). This species of whales is also classified as endangered (1), making them a key focal species for research in our modern times of shifting conditions in ocean environments.

Although I am working to correlate the acoustic detections of fin whales across space and time with environmental drivers (like temperature and chlorophyll concentration), as an aspiring cetacean bioacoustician, one of my other burning related questions is: How can fin whale vocalizations be utilized to differentiate populations across the oceans? Perhaps my analysis of fin whales off the Oregon coast can contribute to the pool of researchers studying this species worldwide to help understand drivers of fin whale vocalization variability.
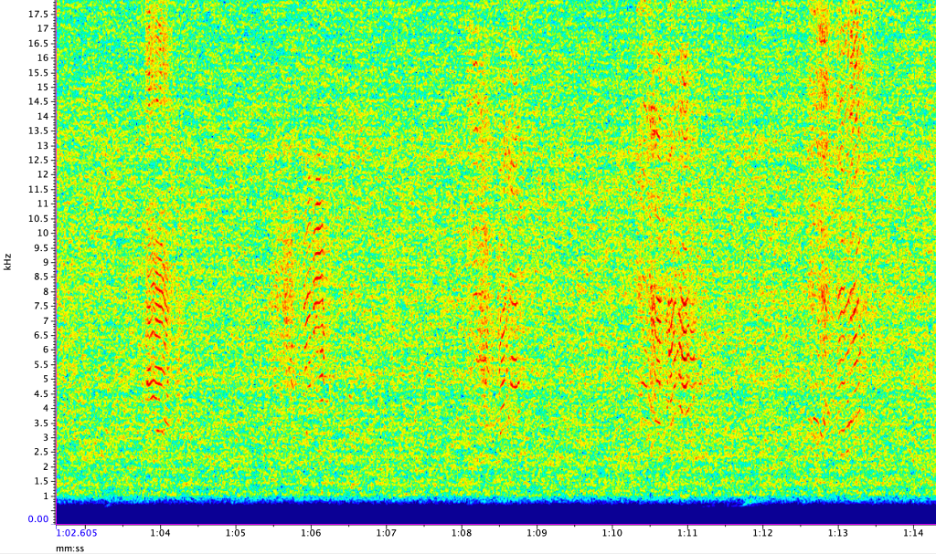
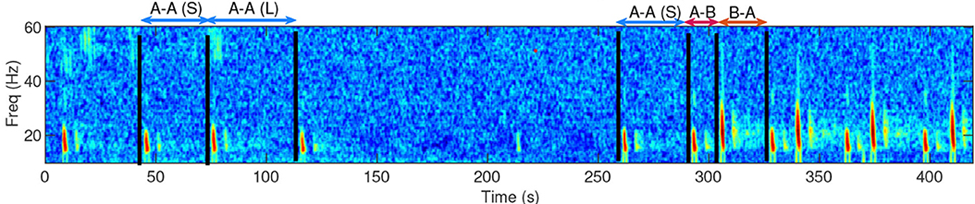
Fin whales can travel great distances, yet their unique vocal renditions of repetitive pulse calls at either a 20 Hz or 40 Hz frequency have geographic patterns (4). These renditions are stereotyped by inter-pulse interval (IPI), which is the rate at which the pulses are detected (5). What’s even more interesting is that unlike many other large baleen whale species, there is little evidence of seasonal behavior and vocalization patterns (5) (Figs. 2 & 3). This suggests that fin whales might not make repetitive annual migrations to accommodate foraging and reproduction. Are these animals prey driven with exemplary senses for finding prey over incredibly large distances in the ocean? Are fin whales consistently present off the Oregon coast? What are their names? Bob, Lucinda, Frederick? There is much to ponder here.

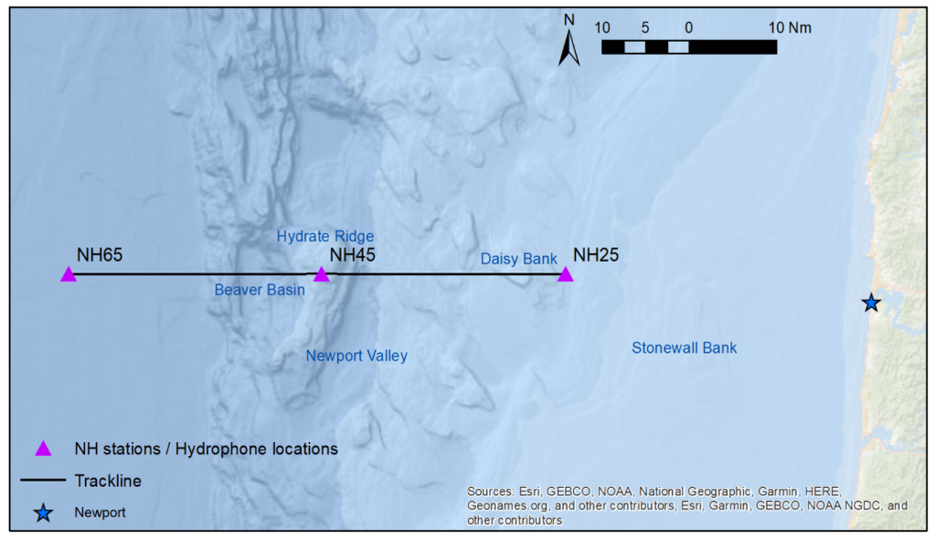
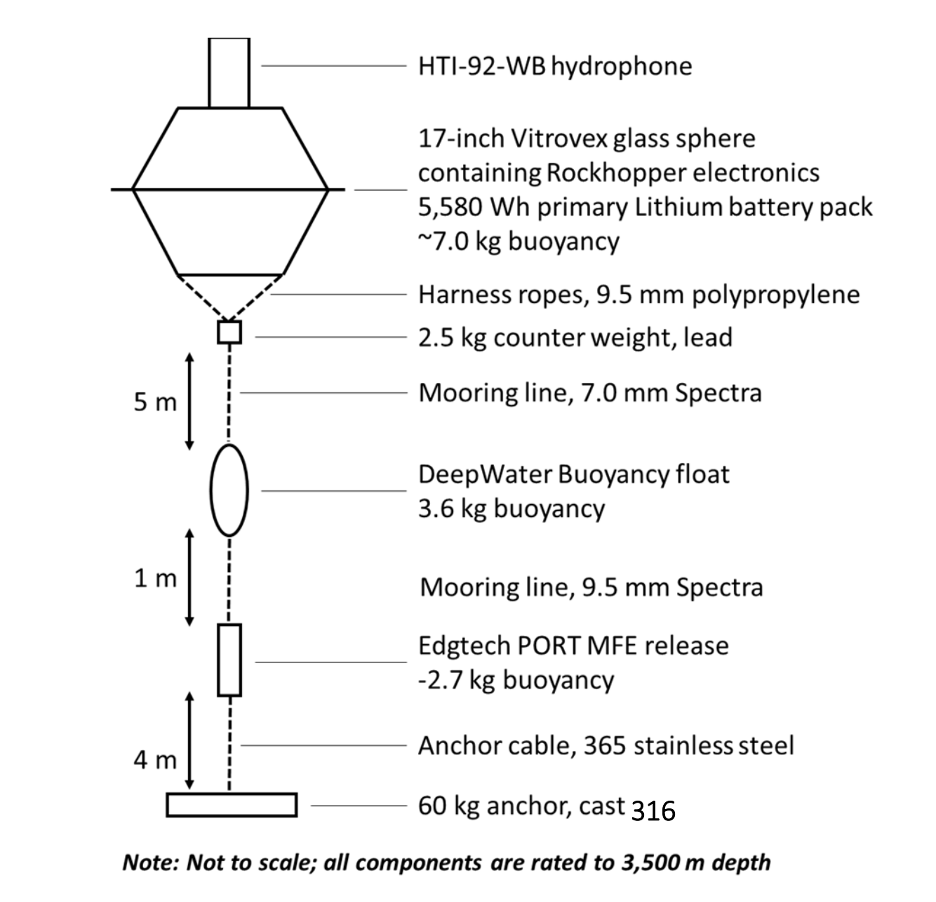
This past summer the Holistic Assessment of Living marine resources off Oregon (HALO) team recovered its first six months of continuously collected acoustic data from three hydrophones moored at designated source locations off the Newport coast. Around the same time, I transplanted my sidekick and myself in Ithaca, New York for the summer, so I could spend my summer days learning to identify and log baleen whale calls among other acousticians at the K. Lisa Yang Center for Conservation Bioacoustics at Cornell University. This work would contribute to my preparation for the analysis of the HALO acoustic data.
I was less than a month into this work when my sidekick ended up spending an entire week with us in the lab because the counselors at her summer camp all caught COVID-19. My sidekick is a dedicated book worm and had no problem keeping herself busy while we all worked, however, she is young and vivacious and so she would often share her music and jokes with the group. I recall (with an uncontrollable smirk on my face) one of her songs called the “Oof” song (Video 1), that is literally a repetitive beat with the onomatopoeia, “oof” being played over and over again. When it started playing I looked up from my computer to see a row of researchers sitting next to Mavis all bobbing their heads to the repetitive tone of “oof”, a tone that hilariously reminded us of a sped-up version of the repetitive pulse of fin whale song. From that point on, “oof” has involuntarily become a part of our language among this group of acousticians.
The summer blazed by, Fall is here, and my sidekick and I are back in Oregon. I am in the process of organizing our collected HALO data to accommodate analysis of baleen whales, including fin whales. At this point I am already able to see fin whale calls in our data (Fig. 4). Subsequently, I will spend the next few months analyzing these data to determine the patterns of fin whale calls over time at our three observation sites (on the shelf, the shelf edge, and off the shelf). Within this analysis I will also look to define the vocal repertoire of fin whales over our six-month study period, which will allow me to report on the frequency where they are primarily detected and the IPI with which the pulses occur.

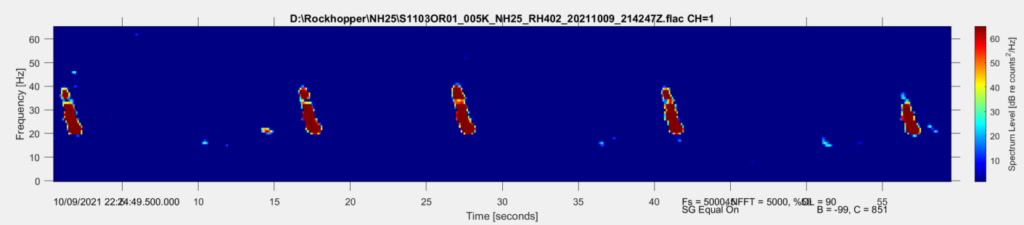
Moving forward, the HALO team will continuously retrieve and replace the three hydrophones to collect our acoustic data, returning a rich long-term dataset of the study area. I am eager to learn whether the fin whale IPI will remain the same in this location or show changes according to shifts in upwelling or seasonally, assuming they remain in the Northern California Current and do not migrate away. I will continue to assess the acoustic patterns of fin whales over the next year to describe their distribution patterns. All the while with the “oof” song stuck in my head and with my vivacious book worm head banging in the background.
Did you enjoy this blog? Want to learn more about marine life, research, and conservation? Subscribe to our blog and get a weekly message when we post a new blog. Just add your name and email into the subscribe box below.
References
(1) Fin Whale. NOAA Fisheries. https://www.fisheries.noaa.gov/species/fin-whale.
(2) Aguilar, A. & Garcia-Vernet, R. 2018. Encyclopedia of Marine Mammals, Third Edition: Fin Whale, Balaenoptera physalus, Pg 369-371. Academic Press, ISBN 978-0-12-804327-1.
(3) Shadwick, R. et al. 2019. Lunge feeding in rorqual whales. Physiology, 34: 409-418. https://journals.physiology.org/doi/epdf/10.1152/physiol.00010.2019.
(4) Oleson, E. et al. 2014. Synchronous seasonal change in fin whale song in the North Pacific. Plos ONE, 9 (12). https://doi.org/10.1371/journal.pone.0115678.
(5) Morano, J. et al. 2012. Seasonal and geographical patterns of fin whale song in the western North Atlantic Ocean. The Journal of the Acoustical Society of America, 132 (1207): 1207-1212. https://doi.org/10.1121/1.4730890.
(6) Helble, T. et al. 2020. Fin whale song patterns shift over time in the central North Pacific. Frontiers of Marine Science, 2 (Marine Megafauna). https://doi.org/10.3389/fmars.2020.587110.
(7) Weirathmueller, M. et al. 2017. Spatial and temporal trends in fin whale vocalizations recorded in the NE Pacific Ocean between 2003-2013. Plos ONE, 12 (10): e0186127. https://doi.org/10.1371/journal.pone.0186127.