By Rachel Kaplan, PhD candidate, Oregon State University College of Earth, Ocean, and Atmospheric Sciences and Department of Fisheries, Wildlife, and Conservation Sciences, Geospatial Ecology of Marine Megafauna Lab
At the beginning of a graduate program, it’s common for people to tell you how quickly the time will pass, but hard to imagine that will really be the case. Suddenly, I’ve been working on my PhD for almost five years, and I’ll defend in just over two weeks. As I look back, I am amazed by how much I have learned and grown during this time, and how all the different parts of my graduate school experience have woven together. I began my program in 2020 with an intense “bootcamp” of oceanographic coursework, and am ending in 2025 with new analytical skills, a few publications, and a ton of new thoughts about whales and the zooplankton krill, the subjects of my research. My PhD work encapsulates all those different elements in an exploration of ecological relationships between baleen whale predators and their krill prey – which I now see as an expression of oceanographic and atmospheric processes.

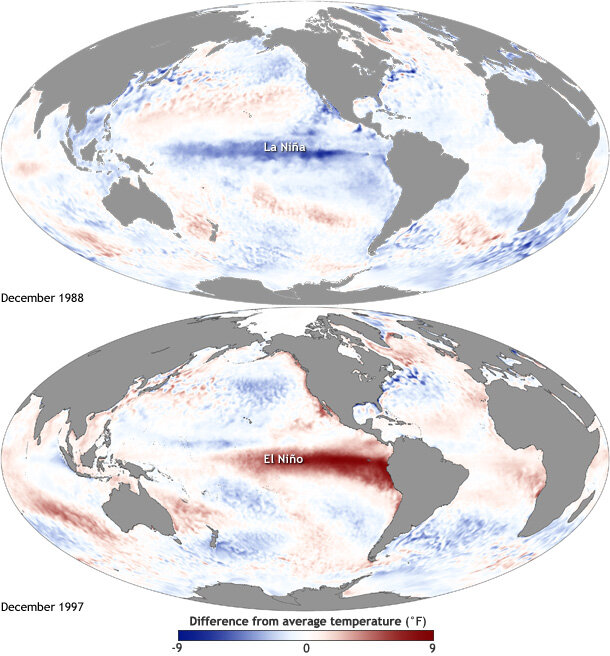
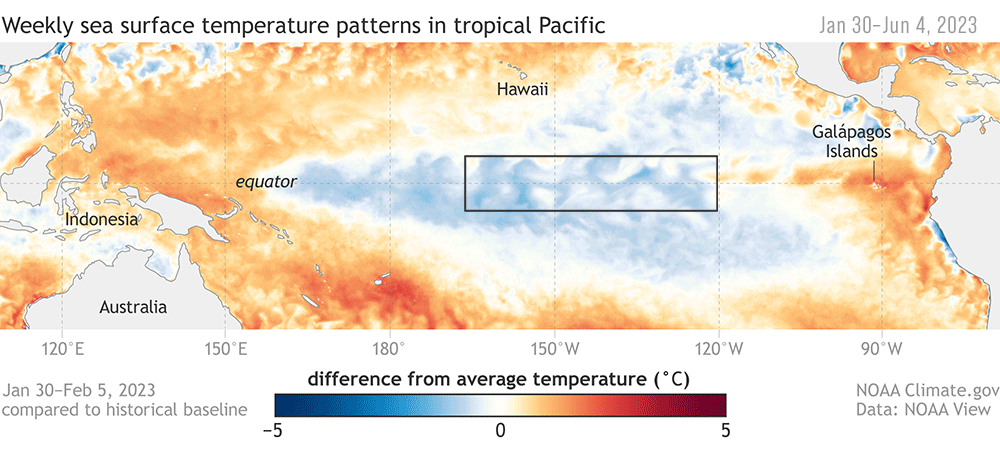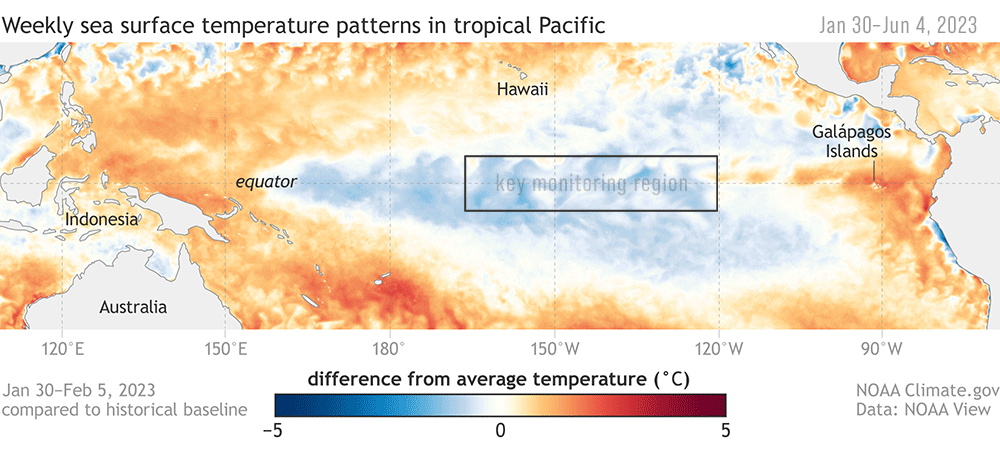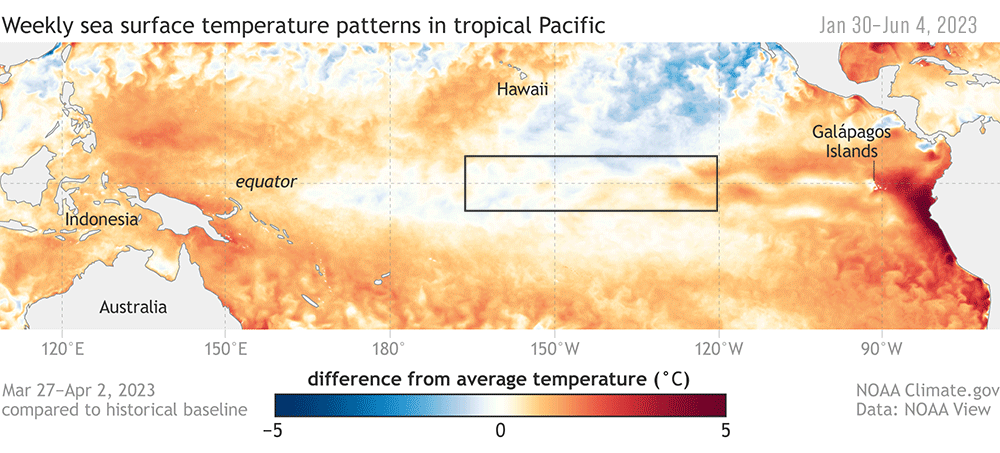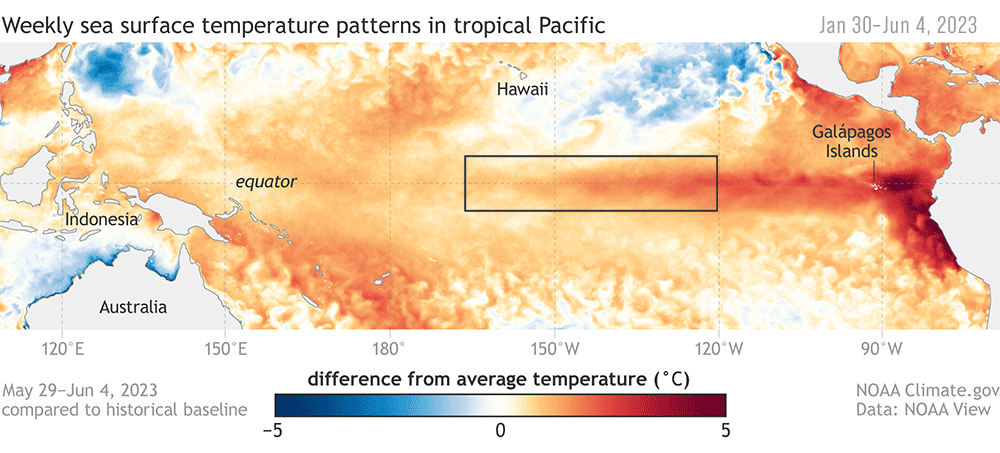
Oceanographic processes drive prey quantity and quality across time and space, shaping the preyscape encountered by predators on their foraging grounds and driving habitat use (Fleming et al., 2016; Ryan et al., 2022). Aspects of prey including distribution, energy density, and biomass therefore represent mechanistic links between ocean and atmospheric conditions (e.g., El Niño Southern Oscillation cycles, circulation patterns, and upwelling processes) and diverse aspects of marine predator ecology, including spatiotemporal distributions, foraging behaviors, reproductive success, population size, and health. Both predator and prey species are impacted by environmental variability and climate change (e.g., Hauser et al., 2017; Atkinson et al., 2019; Perryman et al., 2021), and events like marine heatwaves and harmful algal blooms can force ecosystem changes on short, seasonal time scales (e.g. McCabe et al., 2016; Fisher et al., 2020). However, many marine species have some degree of plasticity that allows them to still accomplish life history events in the face of ecosystem variability (e.g., Lawrence, 1976; Oestreich, 2022), which may provide the capacity to adapt to climate change processes.
Observing and describing predator-prey relationships is complex due to the scale-dependent nature of these relationships (Levin, 1992). Each chapter of my dissertation considered krill, a globally-important prey type, from the perspective of baleen whales, which are krill predators. Chapter 2 used a comparative analysis to identify the optimal spatial scale at which to observe baleen whale-krill relationships on the Northern California Current (NCC) foraging grounds. We found correlations at a 5 km scale to be strongest, which can provide a useful starting point for further studies in the NCC and other systems. Chapter 3 used this spatial scale to compare several aspects of krill prey quality and quantity as predictors of humpback whale (Megaptera novaeangliae) distributions in the NCC. The best performing metric was a species, season, and spatially informed krill swarm biomass variable – yet the comparable performance of a simple acoustic abundance metric indicated that it can act as a reliable proxy for biomass. This finding may be advantageous for future research, as measuring the acoustic proxy is less computationally intensive and relies on fewer datastreams. Interestingly, one of this study’s best-performing models was based on only the proportion of Thysanoessa spinifera in krill swarms, which is also a highly accessible variable due to effective krill species distribution modeling in the NCC (Derville et al., 2024). Integrating the acoustic abundance proxy and krill species distribution predictions, two relatively simple metrics, could support predictions of humpback whale distributions in the NCC and inform whale-prey research in other ecosystems.

Studies relating predator foraging to prey characteristics often rely on metrics such as prey biomass or energy density (Schrimpf et al., 2012; Savoca et al., 2021; Cade et al., 2022), but the tendency of krill to form aggregations introduces dimensionality to krill prey quality. Chapter 4 showed that elements of krill swarm structure (particularly depth, proportion of T. spinifera, and metrics describing how krill occupy space within swarms) may be mechanistic drivers of variable blue, fin and humpback whale distribution patterns on the NCC foraging grounds. These findings suggest that krill swarm characteristics may be important links between baleen whales and the foraging environment. Swarm characteristics may be considered a component of krill prey quality for baleen whales, and future research could illuminate direct causal relationships between oceanographic conditions, krill swarming responses, and niche expression in baleen whale predators.
The relationships between baleen whale distributions and krill quantity and quality explored in the first chapters of my dissertation may also shed light on other aspects of baleen whale ecology. The final chapter considers overwintering trends in global baleen whale populations, and examines the wintertime Western Antarctic Peninsula (WAP) as a case study. Extended humpback whale presence on the WAP feeding grounds may be driven by the profitable feeding areas and elevated energy content of krill during the winter months, and may reflect the high energetic needs of certain demographic subgroups (e.g. lactating females, juveniles). Wintertime humpback whale presence may also reflect adaptation to multifaceted competitive pressure on krill resources that are declining due to climate change (Atkinson et al., 2019), including consumption by growing baleen whale populations (Johnston et al., 2011) and a fishery whose catch limits may be impacting krill predators (Watters et al., 2020; Savoca et al., 2024). This work demonstrates how investigating prey quality during the winter months can contextualize baleen whale overwintering on the foraging grounds. It also provides a meaningful violation of the canonical baleen whale migration paradigm central to marine mammal science, which may lessen the efficacy of whale monitoring programs and management policies.

Management efforts that aim to mitigate risk to whales often hinge on predictive modeling of whale distributions. Species distribution models (SDMs) can provide managers with spatially and temporally explicit predictions of protected species occurrences (Wikgren et al., 2014; Santora et al., 2020), but species distributions in rapidly changing ecosystems are difficult to predict (Muhling et al., 2020). Findings from this dissertation may inform modeling efforts by suggesting meaningful predictor variables for SDMs, such as krill species on the NCC foraging grounds and swarm energy density at the WAP. This work also speaks to meaningful spatial scales for analyzing predator-prey relationships (i.e., 5 km), and relevant elements of temporal variability (e.g., seasonal cycles of krill energy density).
Just as marine predator-prey relationships are shaped by ocean processes, they likewise have consequences for those processes. For example, krill and other zooplankton are capable of generating large-scale mixing that can overcome stratification of water masses and alter water column structure (Noss and Lorke, 2014). Baleen whales influence global carbon cycles due to the huge amount of prey they consume (Savoca et al., 2021; Pearson et al., 2023) and transport important nutrients along the “great whale conveyer belt” during their vast migrations (Roman et al., 2025). Baleen whales seek krill as an essential prey resource on foraging grounds around the globe, and the impact of this trophic interaction scales up, with implications for ecosystem functioning and management. Continued research into the spatiotemporally dynamic relationships between krill and baleen whales improves our understanding of ocean functioning, and can improve our capacity to live as part of this system.
References
Atkinson, A., Hill, S. L., Pakhomov, E. A., Siegel, V., Reiss, C. S., Loeb, V. J., Steinberg, D. K., et al. 2019. Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nature Climate Change, 9: 142–147.
Cade, D. E., Kahane-Rapport, S. R., Wallis, B., Goldbogen, J. A., and Friedlaender, A. S. 2022. Evidence for Size-Selective Predation by Antarctic Humpback Whales. Frontiers in Marine Science, 9: 747788.
Derville, S., Fisher, J. L., Kaplan, R. L., Bernard, K. S., Phillips, E. M., and Torres, L. G. 2024. A predictive krill distribution model for Euphausia pacifica and Thysanoessa spinifera using scaled acoustic backscatter in the Northern California Current. Progress in Oceanography: 103388.
Fisher, J. L., Menkel, J., Copeman, L., Shaw, C. T., Feinberg, L. R., and Peterson, W. T. 2020. Comparison of condition metrics and lipid content between Euphausia pacifica and Thysanoessa spinifera in the northern California Current, USA. Progress in Oceanography, 188.
Fleming, A. H., Clark, C. T., Calambokidis, J., and Barlow, J. 2016. Humpback whale diets respond to variance in ocean climate and ecosystem conditions in the California Current. Glob Chang Biol, 22: 1214–24.
Hauser, D. D. W., Laidre, K. L., Stafford, K. M., Stern, H. L., Suydam, R. S., and Richard, P. R. 2017. Decadal shifts in autumn migration timing by Pacific Arctic beluga whales are related to delayed annual sea ice formation. Global Change Biology, 23: 2206–2217.
Johnston, S. J., Zerbini, A. N., and Butterworth, D. S. 2011. A Bayesian approach to assess the status of Southern Hemipshere humpback whales (Megaptera novaeangliae) with an application to Breeding Stock G. J. Cetacean Res. Manage.: 309–317. International Whaling Commission.
Lawrence, J. M. 1976. Patterns of Lipid Storage in Post-Metamorphic Marine Invertebrates. American Zoologist, 16: 747–762. Oxford University Press (OUP).
Levin, S. A. 1992. The Problem of Pattern and Scale in Ecology: The Robert H. MacArthur Award Lecture. Ecology, 73: 1943–1967.
McCabe, R. M., Hickey, B. M., Kudela, R. M., Lefebvre, K. A., Adams, N. G., Bill, B. D., Gulland, F. M., et al. 2016. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys Res Lett, 43: 10366–10376.
Muhling, B. A., Brodie, S., Smith, J. A., Tommasi, D., Gaitan, C. F., Hazen, E. L., Jacox, M. G., et al. 2020. Predictability of Species Distributions Deteriorates Under Novel Environmental Conditions in the California Current System. Frontiers in Marine Science, 7.
Noss, C., and Lorke, A. 2014. Direct observation of biomixing by vertically migrating zooplankton. Limnology and Oceanography, 59: 724–732. Wiley.
Oestreich, W. 2022. Acoustic signature reveals blue whales tune life‐history transitions to oceanographic conditions. Functional Ecology. https://besjournals.onlinelibrary.wiley.com/doi/10.1111/1365-2435.14013 (Accessed 20 September 2024).
Pearson, H. C., Savoca, M. S., Costa, D. P., Lomas, M. W., Molina, R., Pershing, A. J., Smith, C. R., et al. 2023. Whales in the carbon cycle: can recovery remove carbon dioxide? Trends in Ecology & Evolution, 38: 238–249.
Perryman, W. L., Joyce, T., Weller, D. W., and Durban, J. W. 2021. Environmental factors influencing eastern North Pacific gray whale calf production 1994–2016. Marine Mammal Science, 37: 448–462. Wiley.
Roman, J., Abraham, A. J., Kiszka, J. J., Costa, D. P., Doughty, C. E., Friedlaender, A., Hückstädt, L. A., et al. 2025. Migrating baleen whales transport high-latitude nutrients to tropical and subtropical ecosystems. Nature Communications, 16: 2125. Nature Publishing Group.
Ryan, J. P., Benoit-Bird, K. J., Oestreich, W. K., Leary, P., Smith, K. B., Waluk, C. M., Cade, D. E., et al. 2022. Oceanic giants dance to atmospheric rhythms: Ephemeral wind-driven resource tracking by blue whales. Ecology Letters, 25: 2435–2447.
Santora, J. A., Mantua, N. J., Schroeder, I. D., Field, J. C., Hazen, E. L., Bograd, S. J., Sydeman, W. J., et al. 2020. Habitat compression and ecosystem shifts as potential links between marine heatwave and record whale entanglements. Nat Commun, 11: 536.
Savoca, M. S., Czapanskiy, M. F., Kahane-Rapport, S. R., Gough, W. T., Fahlbusch, J. A., Bierlich, K. C., Segre, P. S., et al. 2021. Baleen whale prey consumption based on high-resolution foraging measurements. Nature, 599: 85–90.
Savoca, M. S., Kumar, M., Sylvester, Z., Czapanskiy, M. F., Meyer, B., Goldbogen, J. A., and Brooks, C. M. 2024. Whale recovery and the emerging human-wildlife conflict over Antarctic krill. Nature Communications, 15: 7708. Nature Publishing Group.
Schrimpf, M., Parrish, J., and Pearson, S. 2012. Trade-offs in prey quality and quantity revealed through the behavioral compensation of breeding seabirds. Marine Ecology Progress Series, 460: 247–259.
Watters, G. M., Hinke, J. T., and Reiss, C. S. 2020. Long-term observations from Antarctica demonstrate that mismatched scales of fisheries management and predator-prey interaction lead to erroneous conclusions about precaution. Scientific Reports, 10: 2314.
Wikgren, B., Kite-Powell, H., and Kraus, S. 2014. Modeling the distribution of the North Atlantic right whale Eubalaena glacialis off coastal Maine by areal co-kriging. Endangered Species Research, 24: 21–31.