By Dawn Barlow, PhD student, OSU Department of Fisheries and Wildlife, Geospatial Ecology of Marine Megafauna Lab
For my PhD, I am using a variety of data sources and analytical tools to study the ecology and distribution of blue whales in New Zealand. I live on the Oregon Coast, across the world and in another season from the whales I study. I love where I live and I am passionate about my work, but I do sometimes feel removed from the whales and the ecosystem that are the focus of my research.

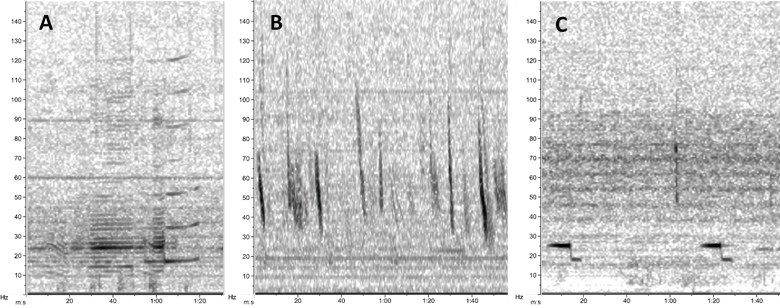
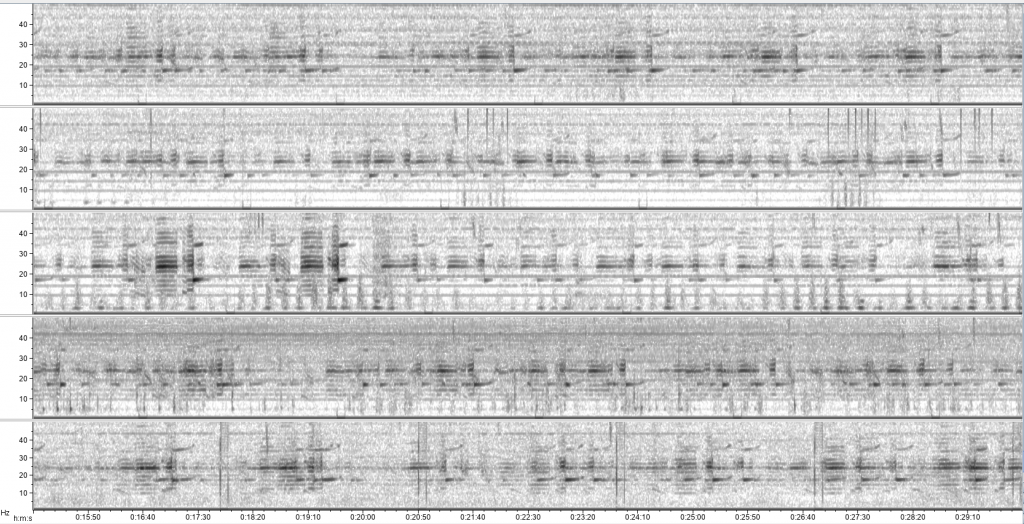
Recently, I have turned my attention to processing acoustic data recorded in our study region in New Zealand between 2016 and 2018. In the fall, I developed detector algorithms to identify possible blue whale vocalizations in the recording period, and now I am going through each of the detections to validate whether it is indeed a blue whale call or not. Looking closely at spectrograms for hours and hours is a change of pace from the analysis and writing I have been doing recently. Namely, I am looking at biological signals – not lines of code and numbers on a screen, but depictions of sounds that blue whales produced. I have to say, it is the “closest” I have felt to these whales in a long time. Scrolling through thousands of spectrograms of blue whale calls leaves room for my mind to wander, and I recently had the realization that those whales have absolutely no idea that on the other side of the Pacific Ocean, there are a few scientists dedicating years of their lives to understand and protect them. Which led me to another realization: we know so much more about blue whales in New Zealand now than we did 10 years ago. In fact, we know so much more than we did even a year ago.

Nine years ago, Dr. Leigh Torres had a cup of coffee with a colleague who recounted observer reports of several blue whales during a seismic survey of the South Taranaki Bight region (STB) of New Zealand. This conversation sparked her curiosity, and led to the formulation of a hypothesis that the STB was in fact an unrecognized feeding ground for blue whales in the southern hemisphere (Torres 2013).

After three field seasons and several years of dedicated work, the hypothesis that the STB region is important for blue whales was validated. By drawing together multiple data streams and lines of evidence, we now know that New Zealand is home to a unique population of blue whales, which are genetically distinct from all other documented populations in the Southern Hemisphere. Furthermore, they use the STB for multiple critical life history functions such as feeding, nursing and calf raising, and they are present there year-round (Barlow et al. 2018).
Once we documented the New Zealand population, we were left with perhaps even more questions than we started with. Where do they feed, and why? Are they feeding and breeding there? Does their distribution change seasonally? What is the health of the population? Are they being impacted by industrial activity and human impacts such as noise in the region? We certainly do not have all the answers, but we have been piecing together an increasingly comprehensive story about these whales and their ecology.
For example, we now know that blue whales in New Zealand average around 19 meters in length, which we calculated by measuring images taken via drones and using an analysis program developed in the GEMM Lab (Burnett et al. 2018). The use of drones has opened up a whole new world for studying health and behavior in whales, and we recently used video footage to better understand the movement and kinematics of how blue whales engulf their krill prey. Furthermore, we know that blue whales may preferentially feed on dense krill aggregations at the surface, and that this surface feeding strategy may be an energetically favorable strategy in this part of the world (Torres et al. 2020).
We have also assessed one aspect of the health of blue whales by describing their skin condition. By analyzing thousands of photographs, we now know that nearly all blue whales in New Zealand bear the scars of cookie cutter shark bites, which they seem more likely to acquire at more northerly latitudes, and that 80% are affected by blister lesions (Barlow et al. 2019). Next, we are beginning to draw together multiple data streams such as body condition and hormone analysis, paired with skin condition, to form a detailed understanding about the health of this population.
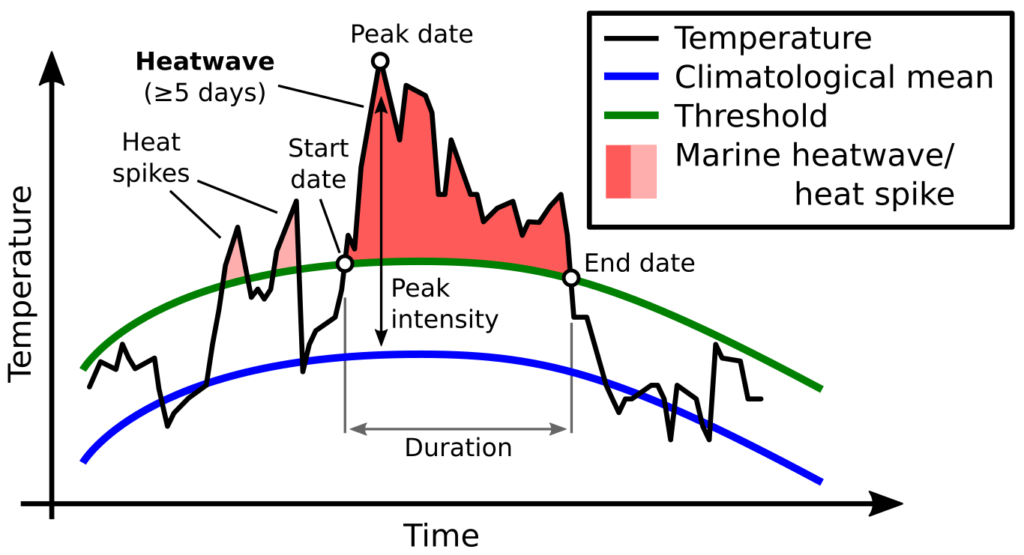
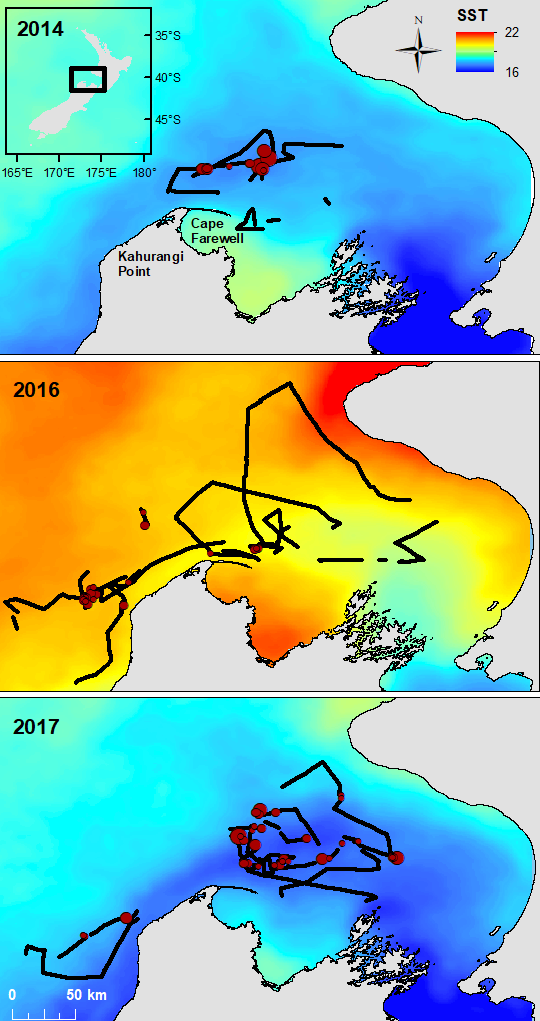
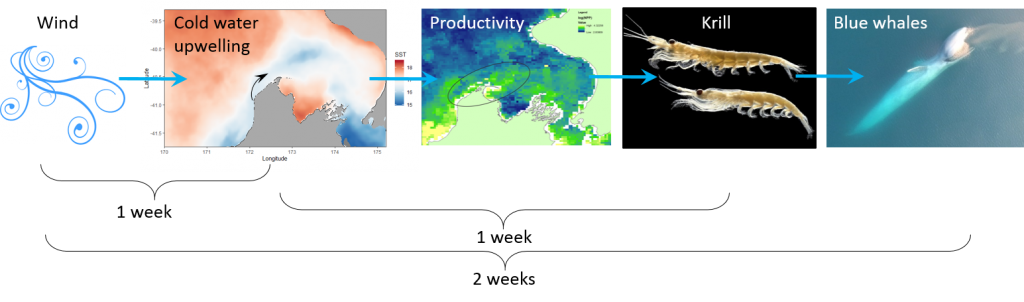
Most recently we have produced a study describing how oceanography, prey and blue whales are connected within this region of New Zealand. The STB region is home to a wind-driven upwelling system that drives productivity and leads to aggregations of krill, which in turn provide sustenance for blue whales to feed on. By compiling data on oceanography and water column structure, krill availability, and blue whale distribution, we now have a solid understanding of this trophic pathway: how oceanography structures prey, and how blue whales respond to both prey and oceanography (Barlow et al. 2020). Furthermore, we are beginning to understand how those relationships may look under changing ocean conditions, with global sea temperatures rising and the increasing frequency and intensity of marine heatwaves.
The knowledge we have accumulated better enables managers to make informed decisions for the conservation of these blue whales and the ecosystem they inhabit. To me, there are several take-away messages from the story that continues to unfold about these blue whales. One is the importance of following a hunch, and then gathering the necessary tools and team to explore and tackle challenging questions. An idea that started over a cup of coffee and many years of hard work and dedication have led to a whole new body of knowledge. Another message is that the more questions you ask and the more questions you try to answer, the more questions you are often left with. That is a beautiful truth about scientific inquiry – the questions we ask drive the knowledge we uncover, but that process is never complete because new questions continue to emerge. Finally, it is easy to get swept up in details, outputs, timelines, and minutia, and every now and then it is important to take a step back. I have appreciated taking a step back and musing on the state of our knowledge about these whales, about how much we have learned in less than 10 years, and mostly about how many answers and new questions are still waiting to be uncovered.

References
Barlow DR, Bernard KS, Escobar-Flores P, Palacios DM, Torres LG (2020) Links in the trophic chain: Modeling functional relationships between in situ oceanography, krill, and blue whale distribution under different oceanographic regimes. Mar Ecol Prog Ser.
Barlow DR, Pepper AL, Torres LG (2019) Skin Deep: An Assessment of New Zealand Blue Whale Skin Condition. Front Mar Sci.
Barlow DR, Torres LG, Hodge KB, Steel D, Baker CS, Chandler TE, Bott N, Constantine R, Double MC, Gill P, Glasgow D, Hamner RM, Lilley C, Ogle M, Olson PA, Peters C, Stockin KA, Tessaglia-hymes CT, Klinck H (2018) Documentation of a New Zealand blue whale population based on multiple lines of evidence. Endanger Species Res 36:27–40.
Burnett JD, Lemos L, Barlow DR, Wing MG, Chandler TE, Torres LG (2018) Estimating morphometric attributes on baleen whales using small UAS photogrammetry: A case study with blue and gray whales. Mar Mammal Sci.
Torres LG (2013) Evidence for an unrecognised blue whale foraging ground in New Zealand. New Zeal J Mar Freshw Res 47:235–248.
Torres LG, Barlow DR, Chandler TE, Burnett JD (2020) Insight into the kinematics of blue whale surface foraging through drone observations and prey data. PeerJ.