Allison Dawn, MSc, GEMM Lab graduate, OSU Department of Fisheries, Wildlife and Conservation Sciences, Geospatial Ecology of Marine Megafauna Lab
The second year of my master’s flew by. Gone were the days of feeling new to graduate school. While I was feeling more comfortable navigating courses, balancing time at both Corvallis and HMSC campuses, and leading recruitment and logistics for the TOPAZ/JASPER field seasons, I certainly felt intimidated by my long, yet exciting, list of research goals I planned to accomplish in order to graduate in Summer 2023. Now, I am proud to say we have come a long way from my (overly ambitious) research proposal and simple Pearson correlations.
At this time last year, I had narrowed down a few key environmental factors to assess relationships between zooplankton in reef systems where PCFG gray whales feed and environmental variability. Even still, I was feeling frustrated at my preliminary analysis results that suggested upwelling had little to no impact on zooplankton abundance or whale foraging effort. This result was dissatisfying given what we know about the upwelling and the California Current System (CCS), so I wondered if my analysis approach, upwelling metrics, or both, were limited. However, thanks to the dedicated mentorship of Leigh, and an informal chat on the water with Aaron Galloway while dive tending for CamDO deployments, I was encouraged to dig even deeper into the literature (and subsequent debates) on the role of upwelling in the nearshore. After several inspiring meetings with the lab about our latest literature deep-dive, I reconfigured my initial hypotheses and charged ahead into the next phases of analysis with a different metric of upwelling than I had calculated before, which I will describe further below. While my final chapter includes a total of seven environmental factors that capture both broad- and fine-temporal temporal scales, for the purposes of this blog I will just share the result of the broad-scale impact of intermittent upwelling on both zooplankton abundance and gray whale foraging effort.
First, a brief recap on upwelling — during the spring and summer in the CCS, strong northerly winds push surface waters offshore, bringing cold, nutrient rich waters from the deep; which creates coastal upwelling. However, upwelling is not persistent. There are periods of time when these northerly winds relax, reducing surface water advection, and upwelling stalls. This relaxation period allows for nearshore retention of primary productivity, which permeates trophic levels with important nutrients. The alternation between upwelling and relaxation is called “intermittent upwelling”, and researchers are finding that the occurrence of relaxation periods are just as important as upwelling itself. Both support biophysical mechanisms that deliver and retain nutrients in the system.
For an example of intermittent upwelling in the CCS, Figure 1 shows a northerly wind stress plot taken from a coastal buoy near our Port Orford study area during 2016. On the y-axis we have northerly wind stress, where positive values show less strong northerly winds, indicating downwelling favorable conditions, and negative values represent strong northerly winds, indicating upwelling favorable conditions. The x-axis is months over time. Here, you can see how in the winter downwelling prevails, but in the summer time we mainly have upwelling favorable winds. However, these summer periods are punctuated by positive values of wind stress, demonstrating that alternations between upwelling and relaxation occur several times throughout the spring and summer period.
Figure 1: Example plot of northerly wind stress plot taken from NOAA Buoy 4601 in 2016 (near our Port Orford, Oregon study area).
The role of upwelling intermittency has been explored in previous work and was posited as the Intermittent Upwelling Hypothesis (IUH) by Menge and Menge 2013. Figure 2, left, demonstrates this hypothesis in theoretical plots. In panel A we see that the rates of ecological processes such as primary productivity and prey response are maximal in at middle values of persistent upwelling and downwelling. In panel B. we see ecological processes positively increase with an index of upwelling intermittency. In Figure 2, right, the authors tested this hypothesis on chlorophyll-a and barnacle and mussel larval recruitment across several study sites and found the results did closely match theory.
Figure 2: Left, Intermittent Upwelling Hypothesis (IUH) theoretical plots showing predicted unimodal relationship between nutrient availability and prey response along a gradient between persistent upwelling and persistent downwelling (panel A) and the expected linear relationship between nutrient availability and upwelling intermittency (panel B); Right, Chlorophyll-a, barnacle, and mussel recruitment responses to an upwelling and intermittency index. Menge and Menge 2013.
Nearshore systems in the CCS, like the ones described in this Menge and Menge 2013 paper, are vastly understudied. And while there is a growing body of literature investigating the role of intermittent upwelling on various prey metrics (Mace & Morgan, 2006; Roegner et al 2007; Benoit-Bird et al., 2019) as well as cetacean movement (Ryan et al., 2022), to our knowledge no study has yet assessed the role of intermittent upwelling on nearshore prey availability and marine mammal occurrence.
To investigate the role of intermittent upwelling, we used the coastal upwelling transport index (CUTI) as our proxy for upwelling. Using daily CUTI values we generated a cumulative upwelling index and number of relaxation events for each year of the study (Figure 3.). This cumulative upwelling information was used to define the day of spring transition (ST) and end of the upwelling season for each year (2016-2021), following the upwelling phenological definitions from Bograd et al. 2009.
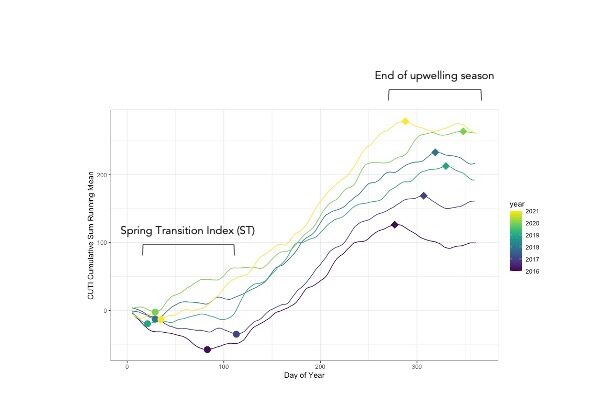
Figure 3: Summed running mean of Cumulative Upwelling Transport Index (CUTI) at latitude 42°N across years 2016-2021, initial data source https://oceanview.pfeg.noaa.gov/products/upwelling/cutibeuti
Using of five-year dataset we investigated functional relationships between each environmental variable and either zooplankton abundance or whale foraging effort using Boosted Regression Tree analysis (Elith et al., 2008). Model results demonstrate that for both zooplankton and whales, species occurrence is high at the intersection between moderate values of accumulated upwelling and with an increasing number of relaxation events. Overall, this work identifies intermittent upwelling as a primary driver of zooplankton abundance and gray whale foraging effort in a nearshore region of Oregon.
Winds in the California Current System are projected to get stronger with climate change, and if upwelling-favorable winds increase in duration and intensity, this could potentially threaten this balance between relaxation and upwelling. While these changes may mean greater primary productivity on some scales, how exactly this increase might affect the very nearshore regions and intermittent upwelling is unknown. Thus, research should continue long-term monitoring of nearshore areas to assist with adaptive management solutions in the face of environmental change.
Preparing this manuscript for my first-first author publication has been another new and exciting process. I feel so grateful for my time as a Master’s student in the GEMM Lab, and for the support of my lab mates, the HMSC community, family, and friends who cheered me on each step of the way to the finish line.
Figure 4: Toasting to a successful master’s defense seminar with GEMM Lab mates, friends and family.
Did you enjoy this blog? Want to learn more about marine life, research, and
conservation? Subscribe to our blog and get a weekly message when we post a new
blog. Just add your name and email into the subscribe box below.
References
Benoit‐Bird, K. J., Waluk, C. M., & Ryan, J. P. (2019). Forage species swarm in response to coastal upwelling. Geophysical Research Letters, 46(3), 1537-1546.
Bograd, S. J., Schroeder, I., Sarkar, N., Qiu, X., Sydeman, W. J., & Schwing, F. B. (2009). Phenology of coastal upwelling in the California Current. Geophysical Research Letters, 36(1).
Curtis Roegner, G., Armstrong, D. A., Hickey, B. M., & Shanks, A. L. (2003). Ocean distribution of Dungeness crab megalopae and recruitment patterns to estuaries in southern Washington State. Estuaries, 26, 1058-1070.
Elith, J., Leathwick, J. R., & Hastie, T. (2008). A working guide to boosted regression trees. Journal of animal ecology, 77(4), 802-813.
Mace, A. J., & Morgan, S. G. (2006). Biological and physical coupling in the lee of a small headland: contrasting transport mechanisms for crab larvae in an upwelling region. Marine Ecology Progress Series, 324, 185-196.
Menge, B. A., & Menge, D. N. (2013). Dynamics of coastal meta‐ecosystems: the intermittent upwelling hypothesis and a test in rocky intertidal regions. Ecological Monographs, 83(3), 283-310.
Oestreich, W. K., Abrahms, B., McKenna, M. F., Goldbogen, J. A., Crowder, L. B., & Ryan, J. P. (2022). Acoustic signature reveals blue whales tune life‐history transitions to oceanographic conditions. Functional Ecology, 36(4), 882-895.
Roegner, G. C., Armstrong, D. A., & Shanks, A. L. (2007). Wind and tidal influences on larval crab recruitment to an Oregon estuary. Marine Ecology Progress Series, 351, 177-188.
Ryan, J. P., Benoit‐Bird, K. J., Oestreich, W. K., Leary, P., Smith, K. B., Waluk, C. M., … & Goldbogen, J. A. (2022). Oceanic giants dance to atmospheric rhythms: Ephemeral wind‐driven resource tracking by blue whales. Ecology Letters, 25(11), 2435-2447.










































