Dr. Alejandro Fernández Ajó, OSU Department of Fisheries, Wildlife, & Conservation Sciences, Geospatial Ecology of Marine Megafauna Lab
Rises in ocean temperatures can lead to multiple alterations in marine ecosystems, including the increase and the frequency of Harmful Algal Blooms (HABs). HABs are characterized by the rapid growth of toxin-producing species of algae that can be harmful to people, animals, and the local ecology, even causing death in severe cases. Species of marine diatom within the genus Pseudo-nitzschia and Nitzschia can form HABs when they produce domoic acid (DA), a potent neurotoxin responsible for amnesic shellfish poisoning (D’Agostino et al., 2018, 2017).

During HABs, DA is transferred to higher organisms through the pelagic food web and is accumulated by intermediate vectors, such as copepods, euphausiids (i.e., krill), shellfish, and fish. As this neurotoxin affects top predators, DA poisoning poses a risk to the safety and health of humans and wildlife. This neurotoxin has caused mortality in many marine mammal species, including both pinnipeds and cetaceans (Gulland 1999; Lefebvre et al. 1999; Fire et al. 2010, 2021; Broadwater et al. 2018). In addition, the exposure to DA constitutes a stressor that may affect glucocorticoids (hormones involved in the stress response) concentrations.
The glucocorticoids (GCs; cortisol and corticosterone) are adrenal steroid hormones that maintain the essential functions of metabolism and energy balance in mammals. GCs can increase sharply in response to environmental stressors to elicit physiological and behavioral adaptations by individuals to support survival (Sapolsky et al. 2000; Bornier et al. 2009). However, with the chronic exposure to a stressor, this relationship can reverse, with GCs sometimes declining below its baseline levels (Dickens and Romero, 2013; Fernández Ajó et al., 2018). Moreover, DA can interfere with the stress response in mammals, and cause alterations in their physiological response. DA is an excitatory amino acid analog of glutamate (Pulido 2008), a well-known brain neurotransmitter that play an important role in the activation of the adrenal axis (which in turn regulate the production and secretion of the GCs) and regulate many of the pituitary hormones involved in the stress response (Brann and Mahesh 1994; Johnson et al. 2001). Hence, monitoring GC levels in marine mammals can be a potential useful metric for assessing the physiological impacts of exposure to DA.
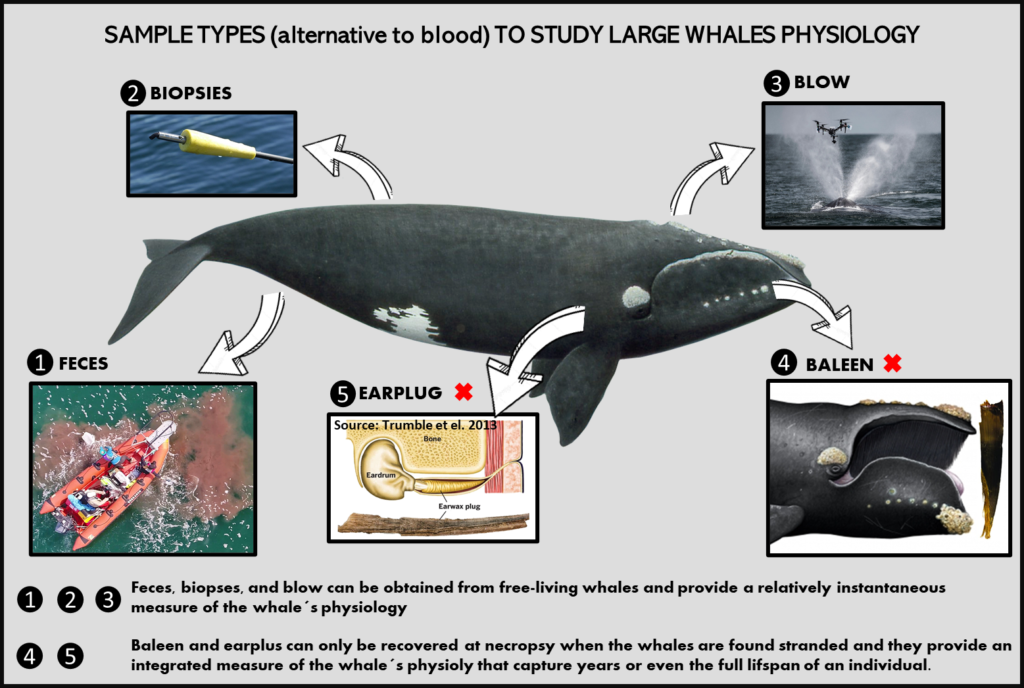
Glucocorticoids are traditionally measured in plasma, but given that plasma sampling from free-ranging large whales is currently impossible, alternative sample types such as fecal samples, among others, can be utilized to quantify GCs in large whales (Ajó et al., 2021; Burgess et al., 2018, 2016; Fernández Ajó et al., 2020, 2018; Hunt et al., 2019, 2014, 2006; Rolland et al., 2017, 2005)(Figure 2). The analyses of fecal glucocorticoid metabolites (fGCm) is particularly useful for endocrine assessments of free-swimming whales, with several studies showing that fGCm correlate in meaningful ways with presumed stressors. For example, high levels of fGCm in North Atlantic right whales (NARW, Eubalaena glacialis) and in gray whales (Eschrichtius robustus) correlate with poor body condition (Hunt et al., 2006; Lemos et al., 2021), and fGCm increases were associated with whale entanglements and ship strikes (i.e., Lemos et al., 2020; Rolland et al., 2017).
In Península Valdés, Argentina, southern right whales (SRW, E. australis) gather in large numbers to mate and nurse their calves during the austral winter months (Bastida and Rodríguez, 2009). SRWs are capital breeders, largely fasting during the breeding season and instead relying on stored blubber fuel reserves. However, they can occasionally feed on calanoid copepods (D’Agostino et al., 2018, 2016), particularly during the phytoplankton blooms that are dominated by diatoms of the genus Pseudo-nitzschia (Sastre et al. 2007; D’Agostino et al. 2015, 2018). Therefore, feeding SRWs in Península Valdés temporally overlap with these Pseudo-nitzschia blooms (D’Agostino et al. 2018, 2015) and represents a test case for assessing the relationship of DA exposure with GC levels (Figure 3).

In our recent scientific publication (D’Agostino et al. 2021), we investigate SRW exposure to DA at their breeding ground in Peninsula Valdes and assessed its effects on fecal glucocorticoid concentrations. Although the sample size of this study is unavoidably small due to the difficulties of obtaining fecal samples from whales at their calving grounds where defecation is infrequent, we observed significantly lower fGCm in samples from whales exposed to DA (Figure 4). Our results agree with findings from a previous study in California sea lions (Zalophus californianus) exposed to DA, where these authors found a significant association of DA exposure with reduced serum cortisol (Gulland et al., 2009), which can be tentatively attributed to abnormal function of the adrenal axis due to the exposure.

If ingestion of toxins produced by phytoplankton can result in long-term suppression of baseline GCs, whales and marine mammals in general, could suffer reduced ability to cope with additional stressors. The adrenal function is essential to maintain circulating blood glucose and other aspects of metabolism within normal bounds. Additionally, the ability to elevate GCs facilitates energy mobilization to physiologically cope with a stressful event and to initiate appropriate behavioral responses (i.e., flee from predators, heal wounds). Various toxicants have been shown to reduce adrenal function across taxa (Romero and Wingfield, 2016) and could have negative consequences on the ability of cetaceans to respond and adapt to ongoing environmental and anthropogenic changes. Compounding this problem, whales are exposed to an increasing number of stressors from multiple sources and with cumulative effects and they need to be able to physiologically respond to continue to reproduce and survive.
To our knowledge, this study provides the first quantification of fGCm levels in whales exposed to DA; and we hope this effort starts a growing dataset to which other researchers can add. Sampling and analysis of non-traditional matrices, such as feces, blubber, baleen and others, would likely increase sample sizes and thus our understanding of the interrelationships among DA exposure and age, sex, and reproductive status of cetaceans. Given that chronic exposure to DA could alter the capacity of animals to respond to stress, and indications that HABs are becoming more frequent and intense world-wide (Van Dolah 2000; Masó et al. 2006; Erdner et al. 2008), we believe that research evaluating the health status of marine mammal populations should include the assessment of stress physiology relative to natural and anthropogenic stressors including exposure to toxicants.
Did you enjoy this blog? Want to learn more about marine life, research, and conservation? Subscribe to our blog and get weekly updates and more! Just add your name into the subscribe box on the left panel.
References
Bastida R, Rodríguez D (2009) Ballena franca austral [Southern right whale]. In: Mazzini V (ed) Mamíferos marinos de Patagonia y Antártida [Marine mammals of Patagonia and Antarctica]. Zagier & Urruty Publications, Buenos Aires, pp 72–84.
Bonier F, Moore IT, Martin PR, Robertson RJ (2009) The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen Comp Endocrinol 163:208–213. https:// doi. org/ 10. 1016/j.ygcen. 2008. 12. 013.
Brann DW, Mahesh VB (1994) Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation. Front Neuroendocrinol 15:3–49. https:// doi. org/ 10. 1006/ frne. 1994. 1002.
Broadwater MH, Van Dolah FM, Fire SE (2018) Vulnerabilities of marine mammals to harmful algal blooms. Harmful Algal Blooms 2:191–222.
Burgess, E.A., Hunt, K.E., Kraus, S.D., Rolland, R.M., 2016. Get the most out of blow hormones: Validation of sampling materials, field storage and extraction techniques for whale respiratory vapour samples. Conserv. Physiol. 4, cow024. https://doi.org/10.1093/conphys/cow024
Burgess, E.A., Hunt, K.E., Kraus, S.D., Rolland, R.M., 2018. Quantifying hormones in exhaled breath for physiological assessment of large whales at sea. Sci. Rep. 8, 10031. https://doi.org/10.1038/s41598-018-28200-8
D’Agostino VC, Hoffmeyer MS, Almandoz GO, Sastre V, Degrati M., 2015. Potentially toxic Pseudo-nitzschia species in plankton and fecal samples of Eubalaena australis from Península Valdés calv-ing ground, Argentina. J Sea Res 106:39–43. https:// doi. org/ 10.1016/j. seares.
D’Agostino, V.C., Degrati, M., Santinelli, N., Sastre, V., Dans, S.L., Hoffmeyer, M.S., 2018. The seasonal dynamics of plankton communities relative to the foraging of the southern right whale (Eubalaena australis) in northern Patagonian gulfs, Península Valdés, Argentina. Cont. Shelf Res. 164, 45–57. https://doi.org/10.1016/j.csr.2018.06.003
D’Agostino, V.C., Degrati, M., Sastre, V., Santinelli, N., Krock, B., Krohn, T., Dans, S.L., Hoffmeyer, M.S., 2017. Domoic acid in a marine pelagic food web: Exposure of southern right whales Eubalaena australis to domoic acid on the Península Valdés calving ground, Argentina. Harmful Algae 68, 248–257. https://doi.org/10.1016/j.hal.2017.09.001
D’Agostino, V.C., Hoffmeyer, M.S., Degrati, M., 2016. Faecal analysis of southern right whales (Eubalaena australis) in Península Valdés calving ground, Argentina: Calanus australis, a key prey species. J. Mar. Biol. Assoc. United Kingdom 96, 859–868. https://doi.org/10.1017/S0025315415001897
Dickens, M.J., Romero, L.M., 2013. A consensus endocrine profile for chronically stressed wild animals does not exist. Gen. Comp. Endocrinol. 191, 177–189. https://doi.org/10.1016/j.ygcen.2013.06.014
Erdner DL, Dyble J, Parsons ML, Stevens RC, Hubbard KA, Wrabel ML, Moore SK, Lefebvre KA, Anderson DM, Bienfang P, Bidi-gare RR, Parker MS, Moeller P, Brand LE, Trainer VL (2008) Centers for Oceans and Human Health: a unified approach to the challenge of harmful algal blooms. Environ Health 7:S2. https://doi. org/ 10. 1186/ 1476- 069X-7- S2- S2.
Fernández Ajó, A., Hunt, K.E., Dillon, D., Uhart, M., Sironi, M., Rowntree, V., Buck, C.L., 2021. Optimizing hormone extraction protocols for whale baleen: tackling questions of solvent:sample ratio and variation. Gen. Comp. Endocrinol. 113828. https://doi.org/10.1016/j.ygcen.2021.113828
Fernández Ajó, A.A., Hunt, K.E., Giese, A.C., Sironi, M., Uhart, M., Rowntree, V.J., Marón, C.F., Dillon, D., DiMartino, M., Buck, C.L., 2020. Retrospective analysis of the lifetime endocrine response of southern right whale calves to gull wounding and harassment: A baleen hormone approach. Gen. Comp. Endocrinol. 296, 113536. https://doi.org/10.1016/j.ygcen.2020.113536
Fernández Ajó, A.A., Hunt, K.E., Uhart, M., Rowntree, V., Sironi, M., Marón, C.F., Di Martino, M., Buck, C.L., 2018. Lifetime glucocorticoid profiles in baleen of right whale calves: potential relationships to chronic stress of repeated wounding by Kelp Gulls. Conserv. Physiol. 6, 1–12. https://doi.org/10.1093/conphys/coy045
Fire SE, Bogomolni A, DiGiovanni RA Jr, Early G, Leighfield TA, Matassa K, Miller GA, Moore KM, Moore M, Niemeyer M, Pugliares K (2021) An assessment of temporal, spatial and taxonomic trends in harmful algal toxin exposure in stranded marine mammals from the US New England coast. PLoS ONE 16(1):e0243570. https:// doi. org/ 10. 1371/ journ al. pone. 02435 70
Fire SE, Wang Z, Berman M, Langlois GW, Morton SL, Sekula-Wood E, Benitez-Nelson CR (2010) Trophic transfer of the harmful algal toxin domoic acid as a cause of death in a minke whale (Balaenoptera acutorostrata) stranding in southern California. Aquat Mamm 36(4):342–350. https:// doi. org/ 10. 1578/ AM. 36.4.2010. 342.
Gulland F.M., 1999. Domoic acid toxicity in California sea lions stranded along the central California Coast, May-October 1998. NOAA Tech. Memo. NMFS-OPR-8. USA National Marine Fisheries Service, US Department of Commerce.
Gulland FMD, Hall AJ, Greig DJ, Fram ER, Colegrove KM, Booth RKN, Wasser SK, S.-M.C., 2009. Gulland, Hall – 2012 – Evaluation of circulating eosinophil count and adrenal gland function in California sea lions naturally exposed t. J. Am. Vet. Med. Assoc. 241, 943–949.
Gulland, F., Pérez-Cortés, H., Urbán, J.R., Rojas-Bracho, L., Ylitalo, G., Weir, J., Norman, S., Muto, M., Rugh, D., Kreuder, C., Rowles, T., 2005. Eastern North Pacific gray whale (Eschrichtius robustus) unusual mortality event, 1999-2000. U.S. Dep. Commer. NOAA Tech. Memo. NMFS-AFSC-150. 33 pp.
Hunt, K.E., Robbins, J., Buck, C.L., Bérubé, M., Rolland, R.M., 2019. Evaluation of fecal hormones for noninvasive research on reproduction and stress in humpback whales (Megaptera novaeangliae). Gen. Comp. Endocrinol. 280, 24–34. https://doi.org/10.1016/j.ygcen.2019.04.004
Hunt, K.E., Rolland, R.M., Kraus, S.D., Wasser, S.K., 2006. Analysis of fecal glucocorticoids in the North Atlantic right whale (Eubalaena glacialis). Gen. Comp. Endocrinol. 148, 260–272. https://doi.org/10.1016/j.ygcen.2006.03.012
Hunt, K.E., Stimmelmayr, R., George, C., Hanns, C., Suydam, R., Brower, H., Rolland, R.M., 2014. Baleen hormones: a novel tool for retrospective assessment of stress and reproduction in bowhead whales (Balaena mysticetus). Conserv. Physiol. 2, cou030–cou030. https://doi.org/10.1093/conphys/cou030
Johnson MP, Kelly G, Chamberlain M (2001) Changes in rat serum corticosterone after treatment with metabotropic glutamate receptor agonists or antagonists. J Neuroendocrinol 13:670–677. https:// doi. org/ 10. 1046/j. 1365- 2826. 2001. 00678.x.
Lefebvre KA, Powell CL, Busman M, Doucette GJ, Moeller PDR, Sliver JB, Miller PE, Hughes MP, Singaram S, Silver MW, Tjeer-dema RS (1999) Detection of domoic acid in northern anchovies and California sea lions associated with an unusual mortality event. Nat Toxins 7(3):85–92. https:// doi. org/ 10. 1002/ (SICI) 1522-7189(199905/ 06)7: 3% 3C85:: AID- NT39% 3E3.0. CO;2-Q.
Lefebvre, K.A., Kendrick, P.S., Ladiges, W., Hiolski, E.M., Ferriss, B.E., Smith, D.R., Marcinek, D.J., 2017. Chronic low-level exposure to the common seafood toxin domoic acid causes cognitive deficits in mice. Harmful Algae 64, 20–29. https://doi.org/10.1016/j.hal.2017.03.003
Lemos, L.S., Olsen, A., Smith, A., Burnett, J.D., Chandler, T.E., Larson, S., Hunt, K.E., Torres, L.G., 2021. Stressed and slim or relaxed and chubby? A simultaneous assessment of gray whale body condition and hormone variability. Mar. Mammal Sci. 1–11. https://doi.org/10.1111/mms.12877
Lemos, L.S., Olsen, A., Smith, A., Chandler, T.E., Larson, S., Hunt, K., Torres, L.G., 2020. Assessment of fecal steroid and thyroid hormone metabolites in eastern North Pacific gray whales. Conserv. Physiol. 8. https://doi.org/10.1093/conphys/coaa110
Masó M, Garcés E., 2006. Harmful microalgae blooms (HAB); prob-lematic and conditions that induce them. Mar Pollut Bull 53:620–630. https:// doi. org/ 10. 1016/j. marpo lbul. 2006. 08. 006.
Pulido O.M., 2008. Domoic acid toxicologic pathology: a review. Mar Drugs 6(2):180–219. https:// doi. org/ 10. 3390/ md602 0180.
Rolland, R., McLellan, W., Moore, M., Harms, C., Burgess, E., Hunt, K., 2017. Fecal glucocorticoids and anthropogenic injury and mortality in North Atlantic right whales Eubalaena glacialis. Endanger. Species Res. 34, 417–429. https://doi.org/10.3354/esr00866
Rolland, R.M., Hunt, K.E., Kraus, S.D., Wasser, S.K., 2005. Assessing reproductive status of right whales (Eubalaena glacialis) using fecal hormone metabolites. Gen. Comp. Endocrinol. 142, 308–317. https://doi.org/10.1016/j.ygcen.2005.02.002
Romero, M.L., Wingfield, J.C., 2016. Oxford series in behavioral neuroendocrinology. Tempests, poxes, predators, and people: stress in wild animals and how they cope 1–2.
Sapolsky RM, Romero LM, Munck AU, 2000. How do glucocorti-coids influence stress response? Intergrating permissive, suppres-sive, stimulatory, and preparative actions. Endocr Rev 21:55–89. https:// doi. org/ 10. 1210/ edrv. 21.1. 0389.
Sastre V, Santinelli N, Marino G, Solís M, Pujato L, Ferrario M., 2007. First detection of domoic acid produced by Pseudo-nitzschia spe-cies, Chubut coastal waters, Patagonia, Argentina. Harmful Algae News 34:12–14.
Van Dolah FM., 2000. Marine algal toxins: origins, health effects, and their increased occurrence. Environ Health Perspect 108:133–141. https:// doi. org/ 10. 1289/ ehp. 00108 s1133
