This week on Inspiration Dissemination, we are looking forward to chatting with Andrew Carpenter, a postdoctoral fellow working in the lab of Professor Joe Baio in the School of Chemical, Biological, and Environmental Engineering.
Andrew’s research seeks a better understanding of a protein called dysferlin, which plays a critical role in repairing muscle cells. Muscles undergo constant strain as they expand and contract, leading to tears in the sarcolemmas — thin membranes that surround muscle fibers. Dysferlin is responsible for recruiting vesicles to the site of these tears for a process called vesicle fusion to take place. Andrew likens this mechanism to using a denim patch to fix a hole in jeans, if the patch could become fully absorbed into the fabric in the way that vesicles eventually do into sarcolemmas. Dysferlin is clinically important because certain mutations (dysferlinopathies) to the gene encoding dysferlin lead to a disease called muscular dystrophy. The symptoms of dysferlinopathy typically include muscle weakness and damage to the musculoskeletal system, especially in the limbs.
The general importance of dysferlin to cell repair is well-established, but the molecular details of its mechanism of action are relatively unknown. Andrew uses an advanced experimental method called sum-frequency spectroscopy to study the protein at high resolution. This procedure uses two lasers — one infrared and one visible green — and points them at the sample of interest. When the lasers hit the sample, a third beam of light is generated at the surface, carrying information about the vibrations of the molecules. Quantum mechanical calculations are used to examine the intensity of this light as a function of frequency. In Andrew’s research, a synthetic lipid monolayer serves as an in-vitro model of the sarcolemma, and he introduces the dysferlin protein either in its healthy form or with various mutations. Then he uses spectroscopy data to infer changes in protein orientation and binding. In the future, he intends to correlate his experiments with data from live cells.
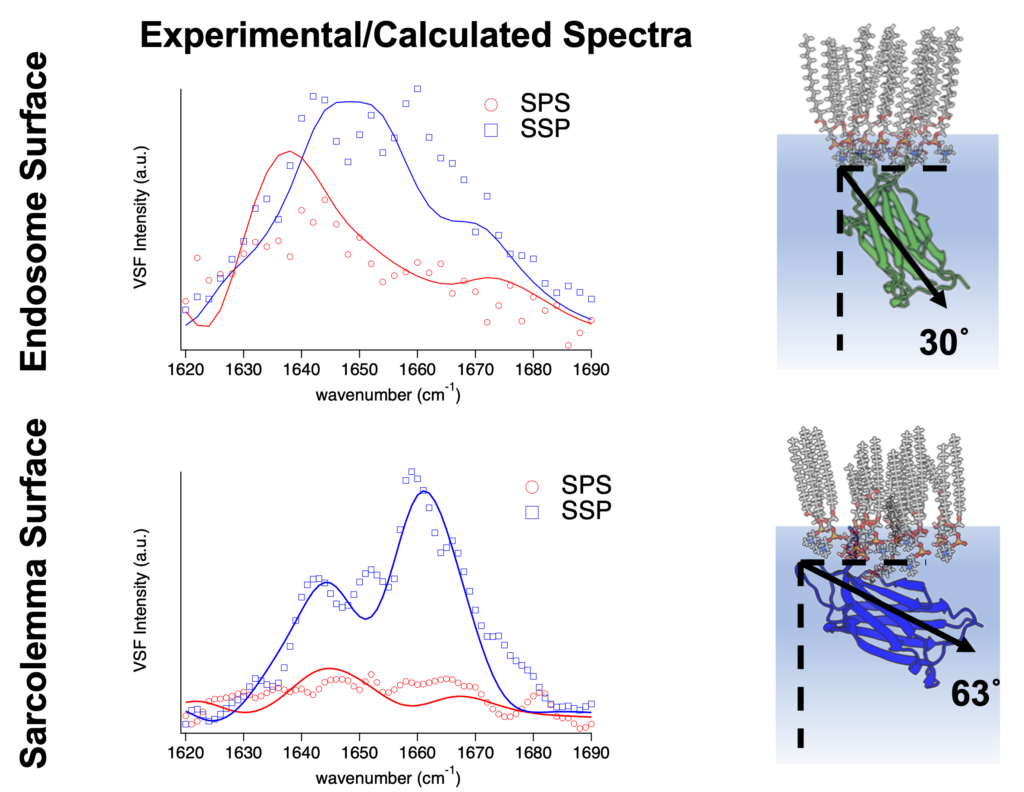

Andrew first discovered his fascination with laser instrumentation as an undergraduate at Linfield University. After that, he obtained a PhD in Chemistry at the University of Oregon, where he used small oil droplets called nano-emulsions to study the oil-water interface. His background in physical chemistry and expertise in the sum-frequency spectroscopy method have enabled him to readily adapt to studying biological lipid interfaces. His research, including a recent publication, is currently supported by the National Science Foundation.
To hear more about Andrew’s research journey and the differences and similarities in being a postdoc and a graduate student, tune in after the Super Bowl this Sunday, February 12th, at 7pm on 88.7 FM KBVR.

