Hello, gardeners! Nina Emond Miller, one of the M.S. graduate researchers in the Garden Ecology Lab, here to share a bit about the science behind the scenes. I hope to give you a glimpse behind the curtain of my research, to highlight the many steps involved in bringing important findings to gardeners like yourself. Today, I will be discussing flies!
Or, perhaps, I should write more ominously… Flies… The bane of many of our existences. They haunt us as fruit flies, house flies, horse flies, and mosquitoes, causing a nuisance and spreading diseases. As a researcher of flies, it is my duty to change that perception and to highlight there is more to these insects! As I wrote in my blog post titled “Calling all gardeners in Corvallis and Portland” in April of 2024, I describe how I became fascinated by flies after reading about their role as pollinators in the Arctic while pursuing my B.S. in Botany. Upon coming across that 2016 paper titled “One Fly to Rule Them All- Muscid Flies are the Key Pollinators in the Arctic” by Tiusanen et al., which described how flies in the house fly family are integral in pollinating Arctic flowers, I wanted to know all about the diversity of pollinating flies. Questions arose as I wondered: To what extent do flies pollinate? What shapes and degrees of fuzziness do these pollinating flies come in? My readings led me to the flower flies, hover flies, or syrphid flies, all common names to describe the Syrphidae family.

Spilogona sanctipauli, one of the most studied Arctic fly pollinators, that belong to the same family as house flies (Image by Malin Ek, 2016).
I find syrphids to be one of the most fascinating families of insects. And though I may be biased in my view of them, there is extensive research highlighting the various roles they serve in ecosystems that also benefit gardeners! As larvae, some species of hover flies can be decomposers, where they process detritus, making essential nutrients such as nitrogen, phosphorus, and potassium available for plants. Without decomposers, our world would be without plants. And of course, a world without plants would be a world without most living creatures.
Alternatively, some species of hover fly larvae are predators. They are impressive consumers of common garden pests such as aphids, thrips, and scale insects! In an article by Dunn et al. (2020), one species was even documented to consume up to 500 aphids throughout their larval stage!

Here is a flower fly larva in action, sucking the fluids out of an aphid (Image credit to Kerry Wixted, 2012).
Most adult syrphids are flower visitors, meaning they are also pollinators. Since they do not have nests that they return to at night or to store food provisions like bees do, they visit flowers daily to consume nectar to properly fuel their everyday lives. Female adult syrphids eat pollen if they are preparing to lay eggs. Protein-rich pollen is essential for proper egg development. By consuming nectar and pollen, they become important pollinators, as they travel between flowers transporting pollen grains as they go.

A commonly observed syrphid here in Oregon called the Narcissus bulb fly visiting a buttercup flower (Image credit to Sandy Rae, 2010).
I met Dr. Gail Langellotto a few years after coming across that article about Arctic flower pollination and she shared my excitement! She brought me on as a M.S. graduate researcher in 2024. Since then, I have dedicated the past year and a half of my life to the syrphids. We wanted to explore what species of hover flies were in urban gardens. We didn’t want to just become familiar with the species, we also wanted to know how plant selection may have influenced what types of hover flies we would see in these gardens. We were also curious to see if garden composition, and surrounding landscapes, potentially influenced which hover fly species we would see.
To begin answering these questions, we recruited 30 gardeners to participate in our study, with 15 gardens in the Portland-metro area, and 15 gardens in Corvallis, OR. We had a great mix of 26 residential gardens and 4 community and demonstration gardens. Our gardens had varying levels of maintenance, tree density, cultivated space, and plant diversity. Once we selected our gardens, we spent the summer of 2024 collecting hover flies in each garden. We sampled over the course of 12 weeks, and we collected 792 syrphids! For each syrphid that we collected from a flower, we noted the plant genus. By doing so, we created a plant list associated with syrphid abundance and syrphid species richness (that is, how many different species were collected from one plant genus). You can read more about our hover fly-friendly plant findings in our Garden Ecology Lab Brief article titled “Garden Plants for Hover Flies”.

Here is Lilly, who recently received her B.S. and a valuable researcher in our lab, expertly collecting hover flies last summer (Image credit to Leah Puhlman, 2024).
Between the summer of 2024 and the summer of 2025, I was lucky enough to collaborate with a brilliant local fly taxonomist of the Pacific Northwest Diptera Research Lab, Dr. Woody Fitzgerald, to identify the 792 hover flies that we collected. Identification is an engaging and thorough process that requires familiarity with insect anatomy, identification lingo, and what species are present in our region. In the end, we identified 28 different species. You can also read more about the 28 species we collected in our second Garden Ecology Lab Brief on hover flies titled “Common Hover Flies in Oregon Gardens”.

Here I am examining my insects, ensuring that I have properly identified them.
So how are we going to go about answering one of our questions: did garden design and the surrounding landscape influence what hover flies we collected in our 30 gardens last summer? That was our task this summer. We first had to get garden-level measurements. We returned to each of our gardens to measure the area of 5 different land use types: permeable, cultivated, impermeable, aquatic, and wood piles. “Permeable” included lawn, soil, mulch, and bark chips. “Cultivated” included ornamental garden beds, edible garden beds, and hedgerows. “Impermeable” included buildings or concrete. “Aquatic” included water features and swampy habitats. “Wood Piles” covered wood that was organized and would be burned within the year. Our goal was to see how different ratios of these land use types influenced the syrphid communities we collected.
We looked at satellite images to develop preliminary garden maps using shapes to most accurately capture each land use type. Then, we visited our gardens, and we took the maps to carry out the measurements. Often, the satellite images did not capture the gardens, either from trees blocking satellite views or if the images were old. One great example comes from one of our community gardens! Between the time that the satellite image was taken and measuring our different areas of the garden, they had added another shed and area “28” had grown into area “14.”
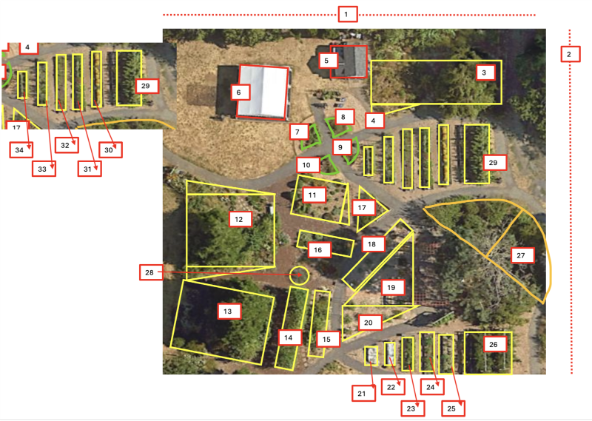
An example of a garden map, with rectangles, trapezoids, triangles, circles, and a semi-ellipse marked to get area measurements for each land use type.
I want to now walk you through the steps we used to gather the garden-level data. For this, we will use a hypothetical example at OSU’s Oak Creek Center for Urban Horticulture. First, we measure the entire property as seen in step 1. Here, we will measure distance “A” and distance “B” to get the total property area.

Step 1: we measure length and width of the entire garden.
We will then define the major land use type. Since grass and bare soil were the major land use types seen in this satellite image of Oak Creek, we would say that “permeable” is our major land use type. We will subtract all other land use type areas from the total area to get the area of “permeable” land use.
In step 2, will measure all areas of “cultivated” space. In the image of step 2, we will measure the length and width of rectangles “C,” “D,” “E,” and “F” to get the total area of “cultivated” space.
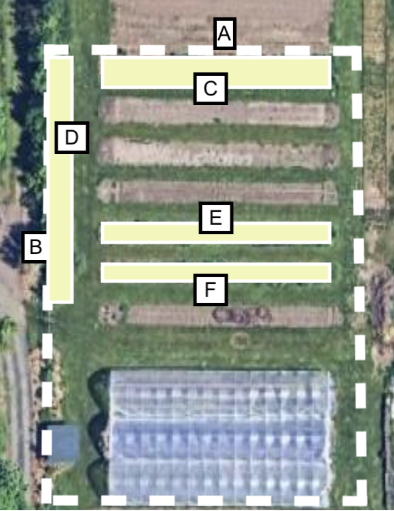
Step 2: we measure the length and width of each individual “cultivated” space.

Here is a picture of Georgia (on the right) and me, measuring the length of a cultivated garden bed at one of our gardens (Image credit to Anna Janowski, 2025).
In step 3, we will measure the area of “impermeable” space. For this example, we will say that the hoop house seen in “G” is “impermeable.” To get the total area of “impermeable” space, we will measure the area of “G” and “H.”
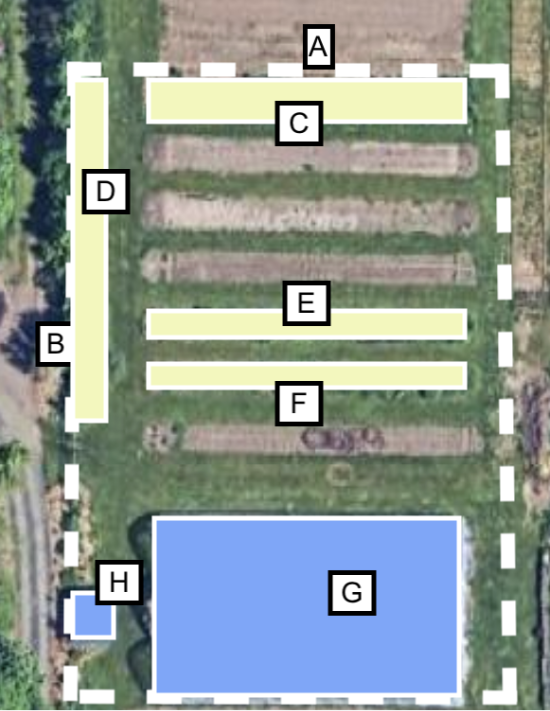
Step 3: we measure the area occupied by “impermeable” space.
Now that we have all of our area measurements, we will examine the ratios using a diversity index measurement. Diversity indices are frequently used in monitoring biodiversity, and they are mostly used on animals and plants. Diversity indices use both abundance (that is how many of one species) and species richness (that is, how many species) to provide a numeric value of biodiversity. For our task here, we will use a diversity index to measure different land use types and its abundance. Our “abundance” will be area measurements, and our “species richness” will be the number of land use types. An area with more diversity in land use types would have a higher diversity index. We will also be able to see if more “cultivated” space in a garden is correlated with hover fly species richness and abundance.
Next, we will be looking at how surrounding habitat influenced what types of hover flies we collected in each garden. To do this, we needed a researcher who was familiar with extracting land use information from satellite images. I am lucky enough to have just the researcher on my graduate committee. Dr. Brittany Barker, a senior researcher and associate professor here at OSU, is an ecologist who codes models. Many of the ecological models that she develops are used by farmers and conservationists to predict pest and invasive species’ presence, allowing them to make informed decisions about pest control.
To answer the question of whether a greener surrounding habitat increased the number and species of syrphids from our gardens, Dr. Barker is developing code to extract information from satellite images about land use types. With each garden as the center point, we will be able to look at the different areas that green surfaces and impermeable surfaces occupy. This will be an interesting way to examine if more urbanized surrounding habitats (e.g., gardens surrounded by more impermeable surfaces) decreased or increased the abundance or species richness of the hover flies that we collected.
While we don’t yet have any answers or patterns to share regarding habitat composition, as that is mine and Dr. Barker’s task for this fall, I hope this highlights the many steps involved in finding answers. Or at the very least, one step towards asking more pointed and informed questions! When I identified hover flies last winter, I kept wondering about the process to create a more local hover fly identification key. Though I graduate at the end of March 2026, I hope to discover a few more patterns and ask more questions about these charismatic flies before then!
References:
Dunn, L., Lequerica, M. Reid, C.R., & Latty, T. (2020). Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest Management Science, 76: 6. https://doi.org/10.1002/ps.5807
Ek, M. (2016). A new use for the old concept of ‘fly paper’ [Image]. Phys Org. Accessed 12 August 2024 from https://phys.org/news/2016-09-flies-key-pollinators-high-arctic.html
Sandy Rae. (2010). Merodon equestris (Large Narcissus fly)- Female [Image]. Wikimedia Commons. Accessed 12 August 2024 from https://commons.wikimedia.org/wiki/File:Merodon_equestris_%28Large_Narcissus_Fly%29_-_female.jpg
Tiusanen, M., Hebert, P.D.N., Schmidt, N.M., & Roslin, T. (2016). One fly to rule them all- muscid flies are the key pollinators in the Arctic. Proceedings of the Royal Society B, 283: 20161271. https://doi.org/10.1098/rspb.2016.1271
Wixted, K. (2012). Syrphid larva noms [Image]. Flickr. Accessed 15 August 2025 from https://www.flickr.com/photos/kwixted0/7946730214





