The Garden Ecology Lab’s Pollinator Plant PR Campaign Presents….. Oregon Sunshine! ☀️
The Garden Ecology Lab is releasing a series of plant profiles of the top 10 Oregon native plants for pollinators, based on Aaron Anderson’s 2017-2019 field trials of 23 Oregon native plants. We will feature one plant per week for 10 weeks, this is week 2! Profiles will include photos, planting information, and will highlight common pollinators of each plant.

Plant Facts
- Scientific Name: Eriophyllum lanatum
- Other names: Common woolly sunflower
- Life Cycle: Perennial
- Foliage: grey, woolly lobed leaves
- Growth Habit: Upright, spreading, “shrubby”; typically 12-14″ in height, may need to be cut back if it becomes too leggy to maintain upright flowers.
- Bloom Duration: June – September
- Hardiness Zone: 5-10; can tolerate cold up to -15 F
- Special Traits: Drought tolerant
- When to plant: Starts can be planted in the spring or fall, seeds should be sown in the fall.
Pollinator Facts
- Oregon Sunshine provides both nectar and pollen to its insect visitors.
- Oregon Sunshine was found to be associated with one species of bee in Aaron’s research: Panurginus atriceps, the black-tipped miner bee.
- Oregon sunshine is a host plant to 7 moths: the Gernaium Plume Moth, Orange Tortrix Moth, the Lupine Ghost Moth, and three moths without common names: Telethusia ovalis, Phalonidia latipunctata, and Phtheochroa aegrana.
- Butterflies including orange sulfurs, red admirals, commas, and skippers are also often attracted to Oregon Sunshine.


Oregon Sunshine‘s Native Range in Oregon
Oregon Sunshine commonly grows on both sides of the Cascades as well as through Southern Washington and California, and has at least 6 different varieties present across the state of Oregon (slide 2). Maps and legend acquired from the Oregon Flora Project, with Imagery Sourced from Google. Copyright 2021© TerraMetrics

Oregon Sunshine as a pollinator plant
Oregon Sunshine is a widespread perennial in the sunflower family (Asteraceae). It provides resources to a great diversity of pollinators, including bees, butterflies, moths, and caterpillars. This native sunflower is a great late summer nectar plant with wide yellow flowers (sometimes up to 2″ across) that allow pollinators easy access to their nectaries!

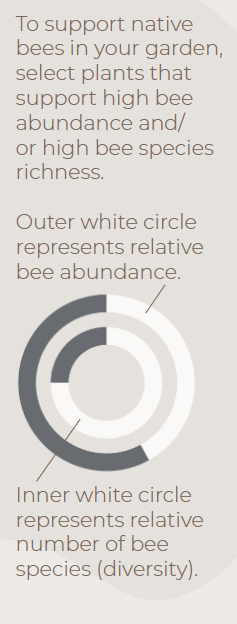
Infographics developed by LeAnn Locher, Aaron Anderson, and Gail Langellotto.
Abundance and Diversity Calculations. Bee abundance was calculated using estimated marginal means of bee visitation to each of our study plants from 5-minute observations conducted from Aaron's 2017-2019 field seasons. Estimated marginal means (EM Means) were assigned to categorical values and averaged across years to yield the following categories: 0% = Very Low =EM mean below 0.49; 25% = Low = EM mean of 0.50 to 0.99; 50% = Moderate = EM mean of 1 to 1.49; 75% = High = EM mean of 1.50 to 1.99; and 100% = Very high = EM mean above 2.0. Bee diversity was based on the total sum of species collected on each of our study plants from 2017 to 2019. A Chao 2 Estimator was used to estimate total expected species richness for each plant; Chao 2 estimates were then used to create categorical values, as follows: 0% = Very Low = 9.99 or lower; 25% = Low = 10 to 14.99; 50% = Moderate = 15 to 19.99; 75% = High = 20 to 24.99; 100% = Very high = 25 or higher.
Did you know?
The white-grey trichomes (the little hairs on the stems and leaves) add a lovely color to gardens and also act as an important adaptation for this drought-tolerant plant. The trichomes help Oregon Sunshine conserve water by both reflecting heat and reducing the amount of air that moves across a leaf’s surface. Though this trait helps Oregon Sunshine endure intense, dry landscapes, it can also explain why it might not do well in the gardens of those with a tendency to “kill with kindness”… this plant does not want a lot of water! It should be watered no more than once a month once established, so over-waterers beware!
Photos from the field
Tune in next week for the next edition of our Pollinator Plant PR Campaign.







































