What’s the first thing people see when approaching a house? The parking strip.
What is often the ugliest, most barren part of a yard? The parking strip!
The parking strip, often called a “Hell Strip”, is a tough landscaping challenge. Narrowly linear, sun-baked, hard to water, often compacted, subject to foot, dog and other traffic…what self-respecting plant would want to grow there?

This is why parking strip “landscaping” tends to default to lawn, mulch, or gravel.
But there’s another option. For every habitat there are plants to match, so if you want a garden in your hell strip, choose plants that LIKE it hot and dry, and are compact in size. Careful design and plant selection can result in a parking strip that is a beautiful asset, rather than a barren wasteland.
As a bonus, many plants that are suitable for planting in a parking strip are also great for pollinators. There are many Oregon native plants that can thrive in such conditions, and since native plants are generally best for pollinators, why not dedicate your parking strip to growing mostly native plants in a beautiful pollinator garden?

Tips for Success
- Before making any parking strip plans, be sure to check with your local government (the owner of the parking strip) for any regulations or requirements you need to take into account.
- Provide a paved landing or path for exiting cars.
- Don’t obscure utility covers with plants.
- Before planting, loosen the soil and dig in compost. It can be worth spending a year or two improving the soil, if it is very bad.
- Plant in fall if possible, to give plants all winter to grow strong roots before having to cope with summer heat and dry.
- Be patient – it may take some trial and error to find the best plants for your parking strip.
Choose the Right Plants
- Low water needs
- Persistent (bulbs, perennials, low shrubs)
- Compact and tidy form
- Attractive foliage
- Variety of textures, shapes and colors
- Varied bloom times over long season
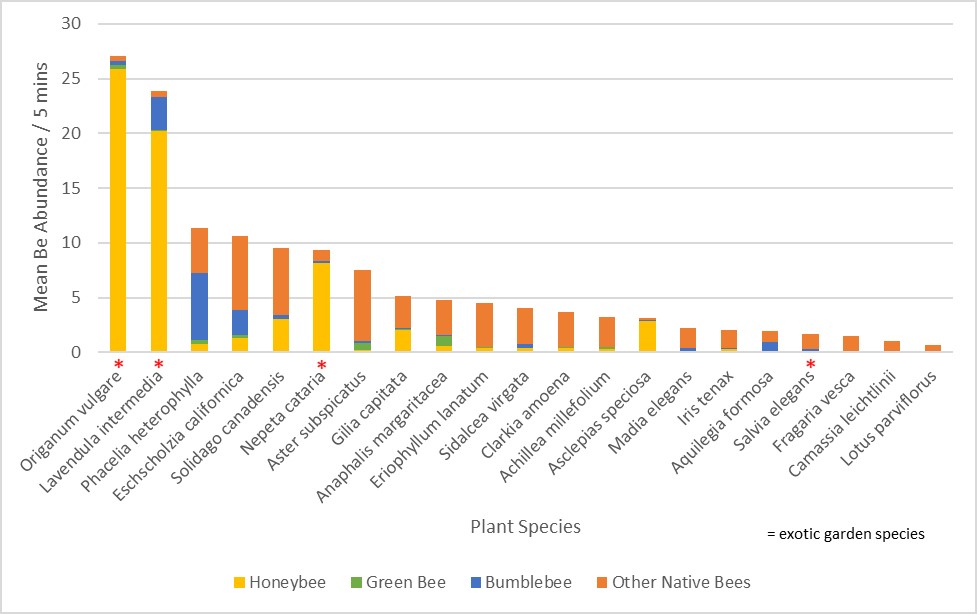
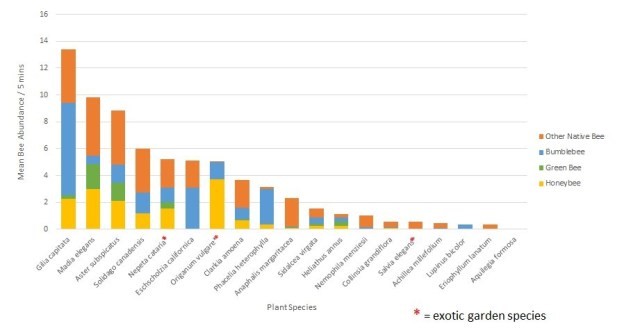
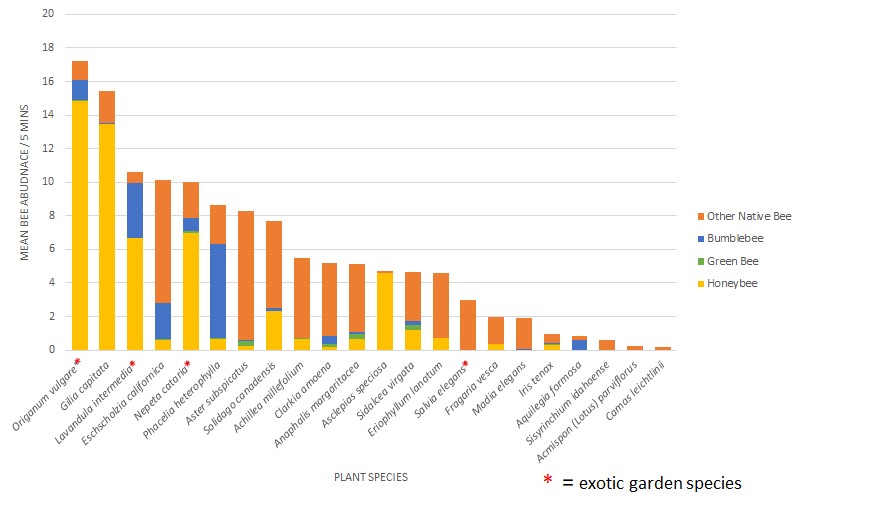
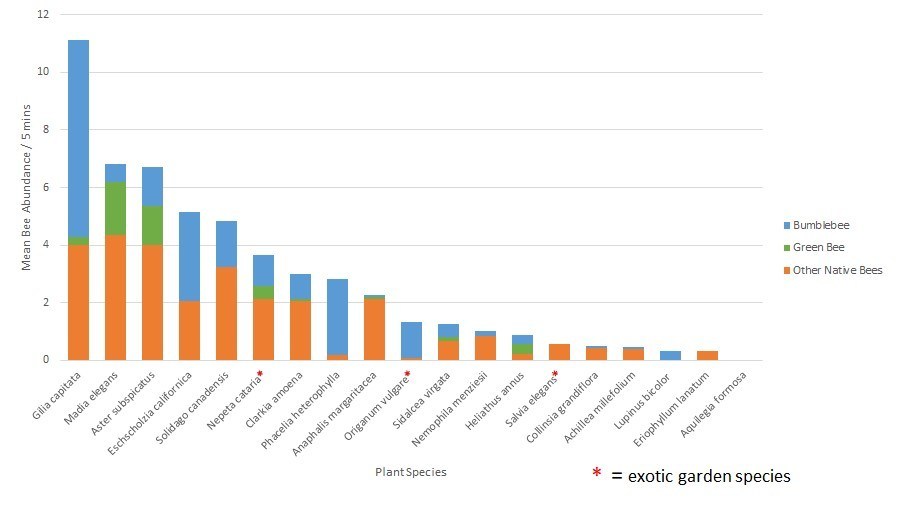
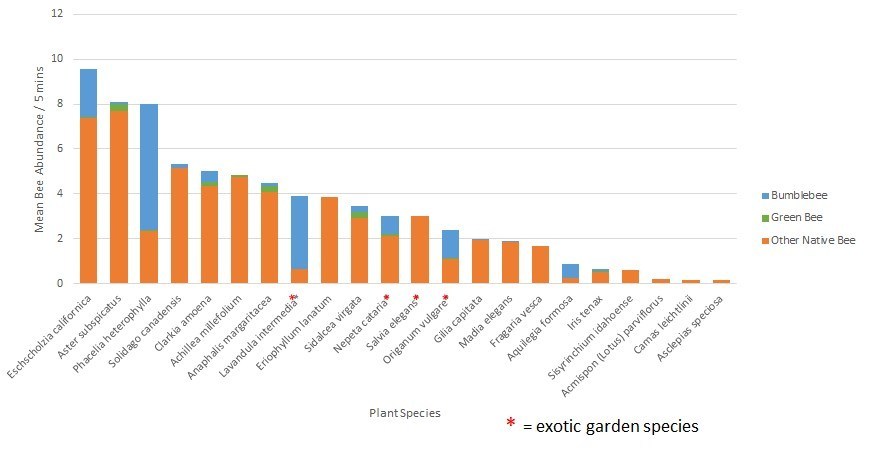
In Jen’s post a couple of weeks ago, http://blogs.oregonstate.edu/gardenecologylab/2020/03/14/how-do-we-know-what-flowers-bees-like/, she listed flower characteristics that bees and butterflies are attracted to. Here’s a short list of plants that feature these characteristics, AND are good candidates for a parking strip planting.

PNW Native Flowers
Achillea millefolium (common yarrow)
Allium cernuum (nodding onion)
Arctostaphylos uva-ursi (kinnikinnick, bearberry)
Balsamorhiza deltoidea (balsamroot, mule’s ears)
Clarkia amoena (godetia, farewell to spring)
Deschampsia cespitosa (tufted hairgrass)
Eriophyllum lanatum (Oregon sunshine)
Eschscholzia californica (California poppy)
Fragaria chiloensis or vesca (beach or woods strawberry)
Gaillardia aristata (blanketflower)
Gilia capitata (globe gilia)
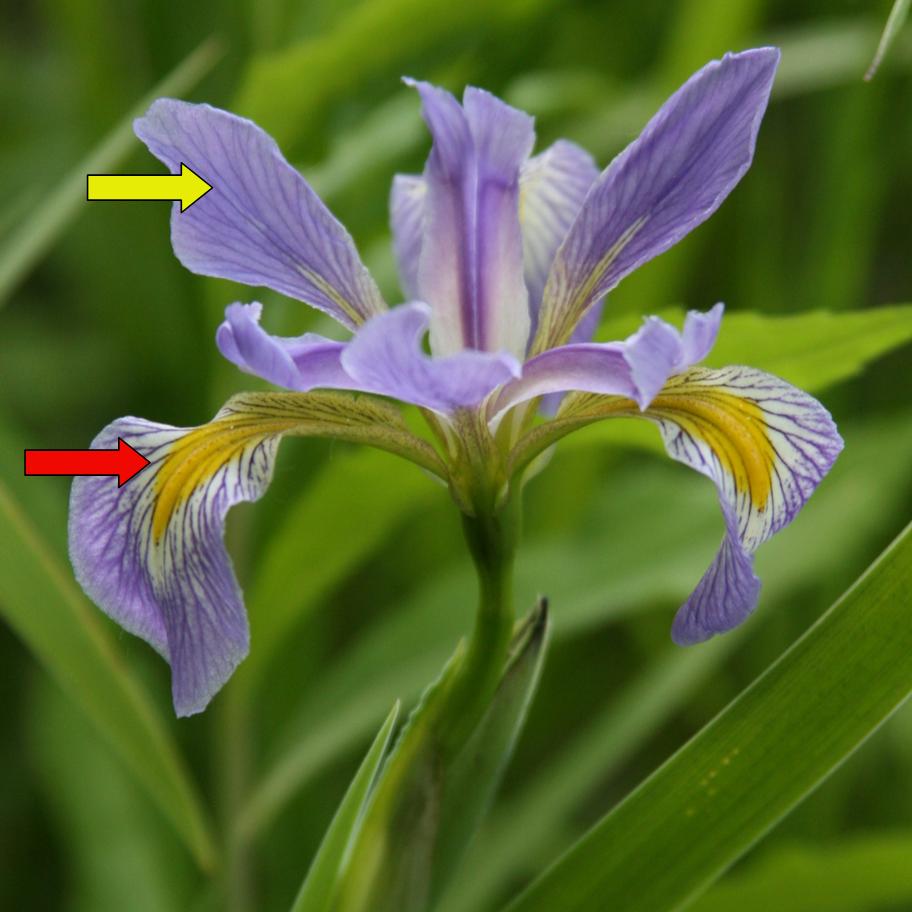
Iris tenax (tough-leaved iris)
Lupinus formosus (western lupine)
Madia elegans (showy tarweed)
Phacelia spp (phacelia)
Plectritis congesta (Seablush)
Sedum spathulifolium ‘Cape Blanco’ (broadleaf stonecrop)
Symphyotrichum/Aster subspicatum (Douglas aster)
You can also add compatible non-native plants, that are also attractive to pollinators.
Bulbs for early bloom: Crocus, Iris reticulata, species tulips
Perennials, Low Shrubs, and Ornamental grasses
Achillea ‘Moonshine’ (yarrow)
Callirhoe involucrata (wine cups)
Caryopteris (blue mist shrub)
Coreopsis grandiflora (largeflower tickseed)
Dianthus ‘Allwoodii’, ‘Flashing Lights’ and others (pinks)
Epilobium (Zauschneria) spp (hummingbird trumpet, Calif. Fuchsia)
Lavandula (lavender)
Nepeta cvs (catmint)
Penstemon spp
Perovskia (Russian sage)
Sedum spp
Thymus ‘Elfin’, ‘Archer’s Gold’ ‘Doone Valley’, red creeping
Resources:
“Hellstrip Gardening” by Evelyn Hadden & Joshua McCullough
“On the Verge” by Tracy Byrne www.pacifichorticulture.org/articles/on-the-verge/
Pollinator Parkways Do-it-Yourself Manual