Alexander Wong, Graduate Research Assistant, Dept. of Botany and Plant Pathology, OSU
Dr. Walt Mahaffee, Research Plant Pathologist, USDA-ARS and FRAME Networks group (USDA-NIFA-SCRI)
The concerning emergence of fungicide resistant grape powdery mildew (Erysiphe necator) to numerous fungicides (Beresford et al. 2016; Cherrad et al. 2018; Colcol et al. 2012; Colcol and Baudoin 2016; Kunova et al. 2016; Miles et al. 2012; Wong and Wilcox 2002) has led to a need for new integrative pest management strategies in grape production. Ultraviolet spectrum C (UVC) radiation has been used for over a century to kill or disable microorganisms by damaging their DNA. However, prolonged exposure times have been required because the microbes have very efficient DNA damage repair machinery (Beggs 2002). A recent discovery (Janisiewicz et al. 2016; Suthaparan et al. 2016) showed that this machinery is shut off at night to conserve energy, which indicates that UVC light applied at night might be effective with shorter exposure times (e.g., strawberry powdery mildew (Onofre et al. 2019), and wheat powdery mildew spores (Zhu et al. 2019)). In collaboration with David Gadoury at Cornell University and Michelle Moyer at Washington State University, we began testing whether UVC could be used to manage grape powdery mildew and bunch rot this past growing season.
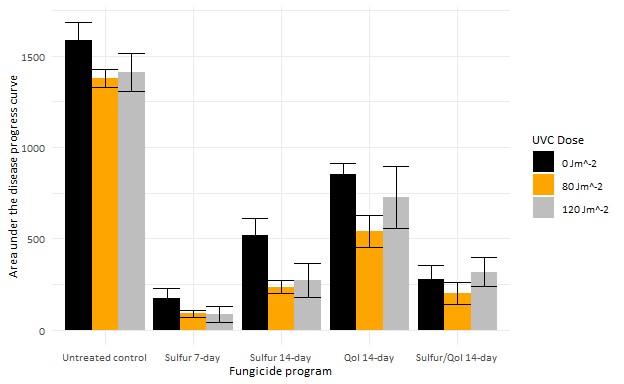
Our research tests were conducted in a 22-year-old block of vertical shoot positioned (VSP) trellised Pinot noir trained at the OSU Botany and Plant Pathology Farm in Corvallis, Oregon using an over-canopy array of UVC light banks (Figure 1). UVC treatments were applied once per week, one hour after sundown at a speed of two or three miles per hour, which relates to a theoretical dose of 120 and 80 joules per square meter (J/m2), respectively. We also applied fungicide programs to subplots within rows of 5 lb/A sulfur on 7- to 14-day intervals, 10 fl oz/A Azoxystrobin on a 14-day interval, an alternation of sulfur and a QoI on a 14-day interval, or untreated control. Powdery mildew incidence ratings were performed every other week starting in mid-May and ending at veraison with leaf and cluster mildew severity ratings completed just before veraison. Grape powdery mildew leaf incidence was significantly (TukeyHSD, p < 0.05) reduced with weekly UV treatments (Figure 2). The UVC treatments did not lead to a significant reduction of mildew severity on clusters, mildew colonies, or rater’s gloves to monitor the amount of mildew (Thiessen et al. 2016) and presence of QoI resistance of each of the plots (Miles et al. 2020).
Botrytis cluster disease severity and incidence was measured at harvest after incubating clusters for 48 hours at 68°F and high humidity. There was no significant difference in Botrytis incidence between treatments. Due to the heavy mildew pressure, many of the clusters with high mildew severity had clusters too desiccated or decayed at harvest to be colonized by Botrytis. Botrytis isolates collected from diseased clusters are being tested for fungicide resistance.
These results suggest that UVC treatments, in conjunction with fungicide programs, has the potential to improve disease management of grape powdery mildew, but the frequency or dose of the application need to be increased. Future field studies at the BPP field site will examine increasing dose and/or UVC application frequency. In collaboration with Willamette Valley Vineyards and Saga Robotics, we will begin exploring the use of an autonomous drive base to apply the treatments on a commercial vineyard scale. Using UVC as part of an integrative pest management tool for powdery mildew will hopefully reduce costs and environmental impacts of disease management by reducing the amount of chemical inputs for disease control.

Literature Cited
Beggs CB 2002. A quantitative method for evaluating the photoreactivation of ultraviolet damaged microorganisms. Photochem Photobiol Sci 1:431-437.
Beresford RM, Wright PJ, Wood PN and Agnew RH. 2016. Sensitivity of grapevine powdery mildew (Erysiphe necator) to demethylation inhibitor and quinone outside inhibitor fungicides in New Zealand. N Z Plant Protec 69:1-10.
Cherrad S, Charnay A, Hernandez C, Steva H, Belbahri L and Vacher S. 2018. Emergence of boscalid-resistant strains of Erysiphe necator in French vineyards. Microbiol Res 216:79-84.
Colcol JF and Baudoin AB. 2016. Sensitivity of Erysiphe necator and Plasmopara viticola in Virginia to QoI Fungicides, Boscalid, Quinoxyfen, Thiophanate Methyl, and Mefenoxam. Plant Dis 100(2):337-344.
Colcol JF, Rallos LE and Baudoin AB. 2012. Sensitivity of Erysiphe necator to demethylation inhibitor fungicides in Virginia. Plant Dis 96(1):111-116.
Janisiewicz, WJ, Fumiomi T, Glenn DM, Camp MJ and Jurick WM. 2016. Dark period following UV-C treatment enhances killing of Botrytis cinerea conidia and controls gray mold of strawberries. Phytopathology 106(4):386-394.
Kunova A, Pizzatti C, Bonaldi M and Cortesi P. 2016. Metrafenone resistance in a population of Erysiphe necator in northern Italy. Pest Manag Sci 72(2):398-404.
Miles LA, Miles TD, Kirk WW and Schilder AMC. 2012. Strobilurin (QoI) resistance in populations of Erysiphe necator on grapes in Michigan. Plant Dis 96(11):1621-1628.
Miles TD, Neill T, Colle M, Warneke B, Robinson G, Stergiopoulos I and Mahaffee WF. 2020. Allele-specific detection methods for Qol fungicide resistant Erysiphe necator in vineyards. Plant Dis.
Onofre RB, Ortiz GA, de Mello Neto PP, Gadoury DM, Stensvand A, Rea M, Bierman A and Peres N. 2019. Evaluation of UVC for suppression of powdery mildew and other diseases of strawberry in open field production. In Technical Abstracts for the American Phytopathological Society (APS) Annual Meeting, Plant Health 2019. Cleveland, OH.
Suthaparan A, Solhaug KA, Stensvand A and Gislerød HR. 2016. Determination of UV action spectra affecting the infection process of Oidium neolycopersici, the cause of tomato powdery mildew. J Photoch Photobio B 156:41-49.
Thiessen LD, Keune JA, Neill TM, Turechek WW, Grove GG and Mahaffee WF. 2016. Development of a grower-conducted inoculum detection assay for management of grape powdery mildew. Plant Pathol 65:238-249.
Wong FP and Wilcox WF. 2002. Sensitivity to azoxystrobin among isolates of Uncinula necator: baseline distribution and relationship to myclobutanil sensitivity. Plant Dis 86(4):394-404.
Zhu M, Riederer M and Hildebrandt U. 2019. UV-C irradiation compromises conidial germination, formation of appressoria, and induces transcription of three putative photolyase genes in the barley powdery mildew fungus, Blumeria graminis f. sp. hordei. Fungal Biol 123(3):218-230.





