Dr. R. Paul Schreiner, Research Plant Physiologist, USDA-ARS, Corvallis, OR
The newest block at Woodhall Research Vineyard is now six years old, and we will begin work in earnest next growing season to ask some fundamental production questions for Pinot noir. The key question is whether opening the top of a standard VSP training system (resulting in a Y-shaped canopy) will increase Pinot noir productivity without sacrificing quality (Figure 1). A second question is whether planting vines at a higher density impacts vine productivity or fruit quality. These questions are being addressed using a factorial experiment where two trellis treatments (traditional VSP & wide VSP) and two vine density treatments (3-foot and 6-foot in-row spacing) are applied in a randomized block design with five blocks. Each experimental plot has five continuous rows of vines about 100 feet long. Data will be collected from the middle three rows, allowing a border row of identical treatment on each side. Different crop levels will be applied to each of the trellis × density treatments by randomly assigning the north or south half of each plot to either low or high crop levels. The trellis and vine density treatments have been in place since 2015, and crop load will be manipulated for the first time next year. The vines were established using industry-standard practices (irrigation, fertilization, no crop in first two years, slowly increasing crop levels thereafter). In the last two years, vines were irrigated only twice each summer, when leaf water potential values reached about -1.4 MPa.
Why this design? Pinot noir producers in western Oregon use a VSP trellis system nearly exclusively where the shoots exist in a tight vertical plane that exposes only a small fraction of leaves to sunlight at midday when solar radiation is maximal. Opening the top of the trellis using a wide VSP system should increase net vine photosynthesis and the vine’s overall carbon budget, allowing more fruit to be produced per acre compared to a traditional VSP. This change can be implemented without removing the existing trellis, keeping costs low for this modification. A similar trellis design was shown to increase yield without compromising quality in Riesling vineyards (Reynolds et al. 1996). Pinot noir producers still thin crop to low levels, leaving 25-40% of their fruit on the vineyard floor. If opening up the canopy can allow Pinot noir producers to ripen more fruit per acre without negatively affecting quality, this approach can increase profits and sustainable production. Vine density per acre may also impact vine productivity or quality directly or by interacting with the altered trellis system. Still, such impacts cannot be predicted based on current knowledge. Since grafted grapevines cost about $5 each, reducing the number of plants needed per acre will significantly reduce establishment costs.
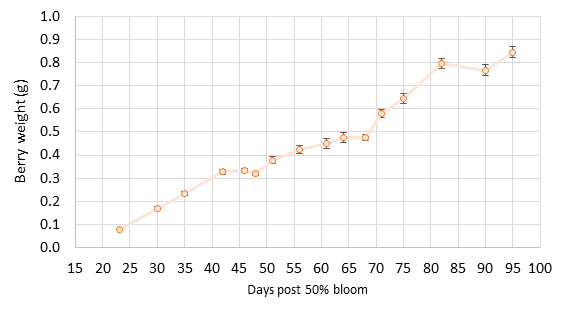
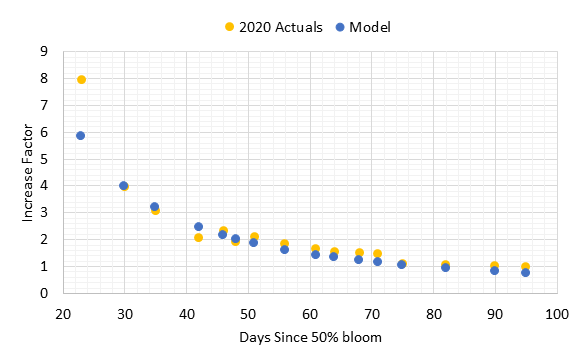
We have collected baseline data from the past five years. The block produced 2.2 US tons per acre in 2019 when the fruit was thinned to one cluster per shoot. Yield in 2020 was 2.5 tons per acre when no fruit thinning was applied due to low set in 2020. Thus far, yield has not been altered by the trellis or vine density treatments. However, vine vegetative growth based on pruning weights was altered for the first time in 2019. The high-density vines produced more shoot biomass in the wide VSP than the traditional VSP, but the low-density vines did not. Thus, the wide VSP appeared to capture more carbon than the traditional VSP in 2019, but only in high-density vines. We do not yet know if a similar response occurred in 2020 since pruning weights have not been obtained yet. Treatments have not altered yield parameters such as cluster weight and berry weight. Fruit composition based on must soluble solids, pH, titratable acids, and mineral nutrient concentrations has not been altered either. The application of different crop levels next year will result in a different yield, and this will begin to provide the true test of this experiment. I am excited to test these ideas on a large scale.
This research addresses improving vineyard production efficiency by altering the most common Pinot noir training system. If our hypothesis is correct, this research will improve Pinot noir wine grape growers’ profitability by increasing yield per acre, thus improving overall land and resource use efficiency.

Literature Cited
Reynolds AG, Wardle DA and Naylor AP. 1996. Impact of training system, vine spacing, and basal leaf removal on Riesling. Vine performance, berry composition, canopy microclimate, and vineyard labor requirements. Am J Enol Vitic 47:63-76.