Well the time has come, the end of my summer internship. It’s been a long summer but I’ve had the chance to learn so much, and I’m so grateful for all the great people I’ve gotten the chance to meet this summer.
Even in my last week I got to experience some new things, during my time here I’ve learned that there’s always more to learn. Monday was nothing too exciting, we processed the Belle Fiore leaves from last week for NSC and then I worked on my report some. On Tuesday we pretty much just stayed in the lab, I set up all the stuff we would need for the rest of the week and in the afternoon Cody and I ran enzymatics on some of the Belle Fiore samples.

Wednesday morning was when the fun really began. We all had to meet up at the station an hour earlier than normal so that we could head over to Cedars, one of our vineyards sites that I had not yet visited. The reason we went over so early in the morning was because at that vineyard site, it’s harvest time! At least for Pinot, which is what our research block consisted of. While we were out there, they had their mechanical harvester going. Essentially the mechanical harvester goes over the vines and hits the row of vines, sending vibrations through it that knock off the grapes. It was pretty cool to watch.

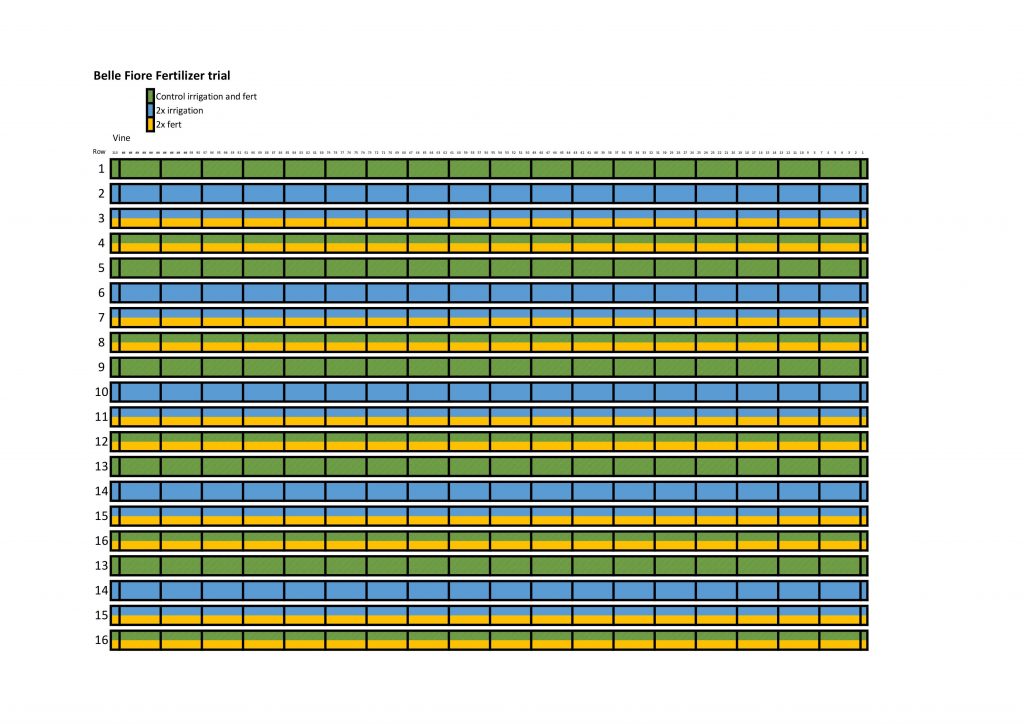
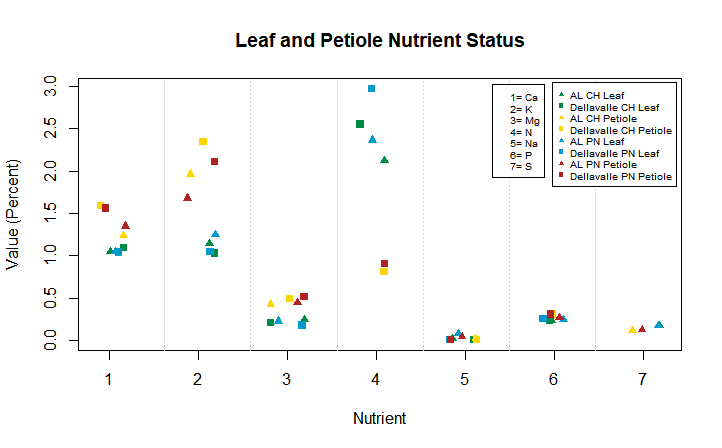
After watching the harvester we got to work and sampled our research block. We subsampled six clusters, two from each central vine, so that we could conduct a cluster analysis, and take measurements of how much the rachis (Stem of the cluster) weighs, and along with some other measurements we can sort of calculate/estimate how much weight is lost due to mechanical harvesting. After that we counted and removed all the clusters from three central vines in each experimental block. We then weighed them and collected them to be sent up to OSU for further analysis.

On Thursday we went out to Redlands and set up the bins, and flagging for harvest as well as cleaned up the vines so when it comes to harvest time it will be a lot easier for us. We also took some berry samples and on Friday morning we ran a juice panel on the samples.
Friday afternoon we had a huge goodbye barbecue for myself and some of the other interns. It was a huge potluck and everyone had an amazing time. It was a great way to say goodbye to all the wonderful people I got to work with over the summer. I want to say thank you to Alexander Levin for letting me work in his lab for the summer and also a thank you to Cody, Joey, Ricky and Reese for making this summer a great internship experience. Also a thank you to the OSU College of Agriculture and the OSU Southern Oregon Research and Extension Center for providing me with this amazing opportunity.