By Lisa Hildebrand, PhD student, OSU Department of Fisheries & Wildlife, Geospatial Ecology of Marine Megafauna Lab
Despite the trials and tribulations of 2020, the GEMM Lab has persevered and experienced many successes and high points. Join me, perhaps with a holiday beverage of choice in-hand, for a summary of what the lab and its members have achieved this year.
The GEMM Lab celebrated several milestones this year. We were all extremely excited and proud when halfway through the year, in July, GEMM Lab PI, Dr. Leigh Torres, was promoted to Associate Professor and granted indefinite tenure in the Department of Fisheries & Wildlife. Leigh joined the department in 2014 and has since completed 13 research projects, is leading 10 current research projects, has graduated 7 graduate students, and is currently advising 4 PhD students and a postdoctoral scholar. A big hurrah to Leigh, our inspiring and tireless captain at the GEMM Lab helm!!
Leigh isn’t the only GEMM Lab member to have received a new title. In March, Leila successfully defended her PhD thesis entitled “Body condition and hormone assessment of eastern North pacific gray whales (Eschrichtius robustus) and associations to ambient noise” and thus graduated from being a PhD candidate to being Dr. Leila Soledade Lemos. Leila is currently a postdoctoral associate at Florida International University. I (Lisa Hildebrand) defended my Master’s thesis “Tonight’s specials include mysids, amphipods, and more: An examination of the zooplankton prey of Oregon gray whales and its impact on foraging choices and prey selection” just a few weeks ago and now bear the title of Master of Science. I am excited to announce that I won’t be leaving the GEMM Lab anytime soon as I will continue to work with Leigh as I pursue my PhD. Our final new title recipient is Dawn who at the start of December advanced to PhD candidacy after successfully passing her written comprehensive exams in mid-November and her oral comprehensive exams in early December.

Leila during her socially-distanced PhD defense seminar. Source: L. Torres. 
Lisa during her Zoom Master’s defense seminar. Source: L. Hildebrand
Summer is a busy time in the GEMM Lab, largely because it is the time when gray whales are distributed along our Oregon coast for their feeding season and therefore when both of our gray whale projects (GRANITE, or Gray whale Response to Ambient Noise Informed by Technology and Ecology, and the Port Orford foraging ecology project) collect another year of data. With the COVID-19 pandemic in its early stages in the spring (when we start to prep for our field seasons), it was uncertain whether we would be able to get into the field at all. However, after weeks of drafting up and submitting COVID-19 safety plans and precautions, Leigh was able to get both of our gray whale field seasons approved to go ahead this summer! This task was not easy since both projects require some form of travel and sampling methods that do not always allow for 6-feet of distance between team members. Furthermore, the Port Orford project requires the whole team to live and work out of OSU’s Port Orford Field Station together. Despite the hurdles, both projects had successful field seasons. If you want to hear more about the specifics of the field seasons, check out the field season summary blog.

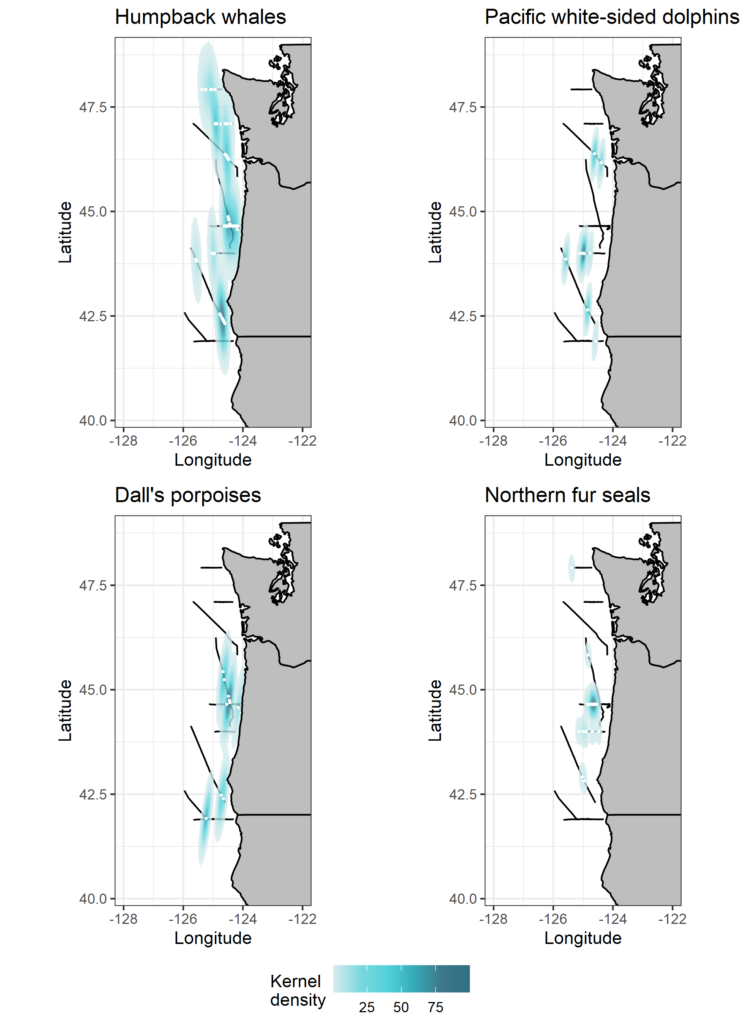
Gray whales weren’t the only species to grab our attention in the field this year. OPAL (Overlap Predictions about Large whales) had a successful second year with Leigh and MMI faculty research assistant Craig Hayslip taking to the skies in United States Coast Guard helicopters four times a month. The project seeks to identify co-occurrence between whales and fishing effort in Oregon to reduce entanglement risk. Leigh and Craig documented numerous cetacean species including blue, fin, humpback, sperm whales, and killer whales. To help with this work, we are so excited to officially have Solène Derville back in the GEMM Lab as a postdoctoral scholar who will work on statistical models aimed at predicting habitat use and distribution patterns of whales off the Oregon coast. While our wish to physically welcome Solène back to Oregon this year did not quite pan out, we are hopeful that she will make the journey from New Caledonia to Oregon in 2021!

The data collected during the helicopter flights will be complimented by the marine mammal observer data that various members of the GEMM Lab have collected over the last four years aboard NOAA Ship Bell M. Shimada as part of the Northern California Current Ecosystem survey. These surveys typically occur three times a year (February, May, September). Although the pandemic threw a wrench into the May cruise, the September cruise was able to go ahead with Dawn and Clara on-board as the two marine mammal observers. It was a very successful cruise, with abundant marine mammal sightings and good survey conditions. Read more about those cruises in Clara and Dawn’s blogs.



While the GEMM Lab did not undertake any field work in New Zealand this year, Leigh and Dawn did travel there in February to meet with scientific colleagues, representatives of the oil and gas industry, and environmental managers, including the New Zealand Minister of Conservation, the Honorable Eugenie Sage. The trip allowed Leigh and Dawn to present their research on blue whales and discuss management implications. These meetings have been highly beneficial as they shared their latest research and results to assist with the development of a marine mammal sanctuary within the industrial region where their research is conducted.



The GEMM Lab prides itself on having strong outreach components to our research, ensuring that young students (high school and undergraduate) from diverse backgrounds have an opportunity to learn STEM skills. Some outreach opportunities were not possible in 2020, but the GEMM Lab continued our efforts where possible. Clara taught a photogrammetry workshop for the Marine Studies Initiative student club Ocean11, where students were taught how to measure whales from drone images. The success of the workshop (and earlier iterations of it in 2019) led to Clara turning it into a lab for Dr. Renee Albertson’s FW 469 Physiology/Behavior of Marine Megafauna class. As one of the program coordinators for the Fisheries & Wildlife Mentorship Program, I co-hosted an Intro to R & RStudio workshop this fall. Rachel taught a remote intensive science communication workshop during her first term in grad school. Although COVID-19 meant that one-on-one mentorships had to be a little more distant, over the course of the year, the GEMM Lab still supervised a total of 7 students that assisted our work in a variety of ways (field and/or lab work, data analyses, independent projects) on a number of projects going on in the lab.
In a typical year, GEMM Lab members would have undertaken quite a lot more travel, largely to attend conferences. Due to COVID-19, most conferences were either cancelled or held virtually. Leigh gave the plenary talk at the annual State of the Coast Conference, one of the favorite conferences of the GEMM Lab as it brings together scientists, stakeholders, managers, students, and the public to discuss Oregon-centric topics. Dawn gave an oral presentation at the International Marine Conservation Congress. The talk was titled “Wind, green water, and blue whales: Predictive models forecast blue whale distribution in an upwelling system to mitigate industrial impacts” as part of a symposium focused on evidence-based solutions for the management of large marine vertebrate species. Clara presented at the annual Research Advances in Fisheries, Wildlife & Ecology symposium hosted by the graduate student association in the Department of Fisheries & Wildlife. Clara’s talk, which was about her proposed PhD research, was titled “Drone footage reveals patterns of gray whale behavior across space, time, and the individual”.
While our travel may have been reduced this year, the lab certainly has had a prolific year of writing! The 19 new publications in 16 scientific journals include contributions from Leigh (6), Leila (5), Rachael (4), Solène (3), Clara (3), Dawn (2), and Ale (1). Scroll down to the end of the post to see the full list.

We are also very excited about a new addition to the lab. Rachel Kaplan, who is co-advised by Leigh and Dr. Kim Bernard in the College of Earth, Ocean, and Atmospheric Sciences, started her PhD at OSU in the fall. Rachel is one of this year’s recipients of the highly-competitive National Science Foundation’s Graduate Research Fellowship. Receiving the fellowship allowed Rachel to wrap up her job at the Bigelow Laboratory for Ocean Sciences in Maine and move to Oregon. The journey wasn’t easy (Rachel moved in the midst of the pandemic and during the height of the wildfires that raged across the U.S. West Coast) but she made it here safely! For her PhD, Rachel will try to understand how oceanographic factors and prey patches shape the distribution of whales in Oregon waters (with data collected through the OPAL project) to work towards solutions to the high rates of whale entanglements in fishing gear that have occurred on the West Coast since 2014. Welcome Rachel!
While we persevered through tough times this year and have been lucky to celebrate many accomplishments, nothing prepared us for the shock that we all felt, and are still feeling deeply, about the loss of our fellow GEMM Lab graduate student Alexa Kownacki just over a month ago. Alexa’s optimism, generosity, and kindness were unparalleled, and the hole that she leaves in the lab and in our lives individually is gaping. The lab wrote a collaborative blog about Alexa a few weeks ago and we have created a website in her honor, where we encourage everyone to post photos, tributes or stories about Alexa. It has been so comforting to us to read people’s memories of Alexa that allow us to learn new things about her and remind us of our own memories. Alexa, we think of you every day and we miss you.

If you are reading this post, we would like to say thank you for all the support and interest in our work – we really appreciate it! Our blog’s viewership this year (a whopping 25,588 views!) has increased over a seven-fold since its creation in 2015 (3,462 views). We hope you will continue to join us on our journeys in 2021. Until then, stay safe, mask up & happy holidays from the GEMM Lab!

Publications
Ajó, A. A. F., Hunt, K. E., Giese, A. C., Sironi, M., Uhart, M., Rowntree, V. J., Marón, C. F., Dillon, D., DiMartino, M., & Buck, C. L. (2020). Retrospective analysis of the lifetime endocrine response of southern right whale calves to gull wounding and harassment: A baleen hormone approach. General and Comparative Endocrinology, 296, 113536.
Albert, C., …, Orben, R. A., et al. (2020). Seasonal variation of mercury contamination in Arctic seabirds: a pan-arctic assessment. Science of the Total Environment, 750, 142201.
Barlow, D. R., Bernard, K. S., Escobar-Flores, P., Palacios, D. M., & Torres, L. G. (2020). Links in the trophic chain: modeling functional relationships between in situ oceanography, krill, and blue whale distribution under different oceanographic regimes. Marine Ecology Progress Series, 642, 207-225.
Baylis, A. M. M., Tierney, M., Orben, R. A., González de la Peña, D., & Brickle, P. (2020). Non-breeding movements of Gentoo penguins at the Falkland Islands. Ibis, doi:10.1111/ibi.12882.
Bird, C., & Bierlich, K.. (2020). CollatriX: A GUI to collate MorphoMetriX outputs. Journal of Open Source Software, 5(51), 2328. doi:10.21105/joss10.21105/joss.02328.
Bird, C., Dawn, A. H., Dale, J., & Johnston, D. W. (2020). A Semi-Automated Method for Estimating Adélie Penguin Colony Abundance from a Fusion of Multispectral and Thermal Imagery Collected with Unoccupied Aircraft Systems. Remote Sensing, 12(22), 3692. doi:10.3390/rs12223692.
Chero, G., Pradel, R., Derville, S., Bonneville, C., Gimenez, O., & Garrigue, C. (2020). Reproductive capacity of an endangered and recovering population of humpback whales in the Southern Hemisphere. Marine Ecology Progress Series, 643, 219-227.
Derville, S., Torres, L. G., Zerbini, A. N., Oremus, M., & Garrigue, C. (2020). Horizontal and vertical movements of humpback whales inform the use of critical pelagic habitats in the western South Pacific. Scientific Reports, 10, 4871.
DiGiacomo, A. E., Bird, C., Pan, V. G., Dobroski, K., Atkins-Davis, C., Johnston, D. W., & Ridge, J. T.. (2020). Modeling Salt Marsh Vegetation Height Using Unoccupied Aircraft Systems and Structure from Motion. Remote Sensing, 12(14), 2333. doi:10.3390/rs12142333.
Garrigue, C., Derville, S., Bonneville, C., Baker, C. S., Cheeseman, T., Millet, L., Paton, D., & Steel, D. (2020). Searching for humpback whales in a historical whaling hotspot of the Coral Sea, South Pacific. Endangered Species Research, 42, 67-82.
Hauser-Davis, R. A., Monteiro, F., Chávez da Rocha, R. C., Lemos, L., Duarte Cardoso, M., & Siciliano, S. (2020). Titanium as a contaminant of emerging concern in the aquatic environment and the current knowledge gap regarding seabird contamination. Ornithologia, 11, 7-15.
Hindell, M. A., … Torres, L. G., et al. (2020). Tracking of marine predators to protect Southern Ocean ecosystems. Nature, 580(7801), 87-92.
Jones, K. A., Baylis, A. M. M., Orben, R. A., Ratcliffe, N., Votier, S. C., Newton, J., & Staniland, I. J. (2020). Stable isotope values in South American fur seal pup whiskers as proxies of year-round maternal foraging ecology. Marine Biology, 167(10), 1-11.
Kroeger, C. E., Crocker, D. E., Orben, R. A., Thompson, D. R., Torres, L. G., Sagar, P. M., Sztukowski, L. A., Andriese, T., Costa, D. P., & Shaffer, S. A. (2020). Similar foraging energetics of two sympatric albatrosses despite contrasting life histories and wind-mediated foraging strategies. Journal of Experimental Biology, 223, jeb228585.
Lemos, L. S., Olsen, A., Smith, A., Chandler, T. E., Larson, S., Hunt, K., & Torres, L. G. (2020). Assessment of fecal steroid and thyroid hormone metabolites in eastern North Pacific gray whales. Conservation Physiology, 8, coaa110.
Monteiro, F., Lemos, L. S., et al. (2020). Total and subcellular Ti distribution and detoxification processes in Pontoporia blainvillei and Steno bredanensis dolphins from southeastern Brazil. Marine Pollution Bulletin, 153, 110975.
Quinete, N., Hauser-Davis, R. A., Lemos, L. S., Moura, J. F., Siciliano, S., & Gardinali P. R. (2020). Occurrence and tissue distribution of organochlorinated compounds and polycyclic aromatic hydrocarbons in Magellanic penguins (Spheniscus magellanicus) from the southeastern coast of Brazil. Science of the Total Environment, 749, 141473.
Soledade Lemos, L., Burnett, J. D., Chandler, T. E., Sumich, J. L., & Torres, L. G. (2020). Intra- and inter-annual variation in gray whale body condition on a foraging ground. Ecosphere, 11(4), e03094.
Torres, L. G., Barlow, D. R., Chandler, T. E., & Burnett, J. D. (2020). Insight into the kinematics of blue whale surface foraging through drone observations and prey data. PeerJ, 8, e8906.